The neurovascular unit and the pathophysiologic basis of diabetic retinopathy
- PMID: 27832340
- PMCID: PMC5551503
- DOI: 10.1007/s00417-016-3548-y
The neurovascular unit and the pathophysiologic basis of diabetic retinopathy
Abstract
Purpose: To relate the concept of the retinal neurovascular unit and its alterations in diabetes to the pathophysiology of diabetic retinopathy.
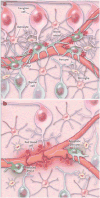
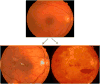
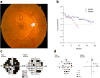
Methods: Case illustrations and conceptual frameworks are presented that illustrate adaptive and maladaptive "dis-integration" of the retinal neurovascular unit with the progression of diabetes.
Results: Retinopathy treatment should address pathophysiologic processes rather than pathologic lesions as is current practice.
Conclusions: Future improvements in the treatment of diabetic retinopathy requires deeper understanding of the cellular and molecular changes induced by diabetes, coupled with the use of quantitative phenotyping methods that assess the pathophysiologic processes.
Keywords: Diabetic retinopathy; Frequency-doubling perimetry; Neurovascular unit; Sensory neuropathy; Spectral domain optical coherence tomography (SD-OCT).
Figures


References
-
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. - PubMed
-
- Lott ME, Slocomb JE, Shivkumar V, Smith B, Gabbay RA, Quillen D, Gardner TW, Bettermann K. Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta ophthalmologica. 2012;90:e434–441. - PubMed
-
- Pemp B, Garhofer G, Weigert G, Karl K, Resch H, Wolzt M, Schmetterer L. Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Investigative ophthalmology & visual science. 2009;50:4029–4032. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

