Weighing Clinical Evidence Using Patient Preferences: An Application of Probabilistic Multi-Criteria Decision Analysis
- PMID: 27832461
- PMCID: PMC5306398
- DOI: 10.1007/s40273-016-0467-z
Weighing Clinical Evidence Using Patient Preferences: An Application of Probabilistic Multi-Criteria Decision Analysis
Abstract
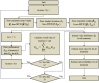
The need for patient engagement has been recognized by regulatory agencies, but there is no consensus about how to operationalize this. One approach is the formal elicitation and use of patient preferences for weighing clinical outcomes. The aim of this study was to demonstrate how patient preferences can be used to weigh clinical outcomes when both preferences and clinical outcomes are uncertain by applying a probabilistic value-based multi-criteria decision analysis (MCDA) method. Probability distributions were used to model random variation and parameter uncertainty in preferences, and parameter uncertainty in clinical outcomes. The posterior value distributions and rank probabilities for each treatment were obtained using Monte-Carlo simulations. The probability of achieving the first rank is the probability that a treatment represents the highest value to patients. We illustrated our methodology for a simplified case on six HIV treatments. Preferences were modeled with normal distributions and clinical outcomes were modeled with beta distributions. The treatment value distributions showed the rank order of treatments according to patients and illustrate the remaining decision uncertainty. This study demonstrated how patient preference data can be used to weigh clinical evidence using MCDA. The model takes into account uncertainty in preferences and clinical outcomes. The model can support decision makers during the aggregation step of the MCDA process and provides a first step toward preference-based personalized medicine, yet requires further testing regarding its appropriate use in real-world settings.
Conflict of interest statement
Compliance with Ethical Standards Author contributions All authors contributed to the study conception and design. HB collected the data from literature and ran the analyses. HB, ABH, and CGMG-O developed the model. HB, MJI, and CGMG-O drafted the manuscript. All authors contributed to the critical revision of intellectual content in the final manuscript. All authors approved the final version for submission. CG is the guarantor for the overall content. Funding No funding from external sources was provided for this work. Conflicts of interest Henk Broekhuizen, Maarten IJzerman, Brett Hauber, and Catharina Groothuis-Oudshoorn declare no conflicts of interest.
Figures

Similar articles
-
Estimating the value of medical treatments to patients using probabilistic multi criteria decision analysis.BMC Med Inform Decis Mak. 2015 Dec 2;15:102. doi: 10.1186/s12911-015-0225-8. BMC Med Inform Decis Mak. 2015. PMID: 26626279 Free PMC article.
-
Two approaches to incorporate clinical data uncertainty into multiple criteria decision analysis for benefit-risk assessment of medicinal products.Value Health. 2014 Jul;17(5):619-28. doi: 10.1016/j.jval.2014.04.008. Epub 2014 Jul 10. Value Health. 2014. PMID: 25128056
-
A scenario-based MCDA framework for wastewater infrastructure planning under uncertainty.J Environ Manage. 2016 Dec 1;183(Pt 3):895-908. doi: 10.1016/j.jenvman.2016.09.027. Epub 2016 Sep 22. J Environ Manage. 2016. PMID: 27666649
-
Strengthening the evidence-base of integrated care for people with multi-morbidity in Europe using Multi-Criteria Decision Analysis (MCDA).BMC Health Serv Res. 2018 Jul 24;18(1):576. doi: 10.1186/s12913-018-3367-4. BMC Health Serv Res. 2018. PMID: 30041653 Free PMC article. Review.
-
A review and classification of approaches for dealing with uncertainty in multi-criteria decision analysis for healthcare decisions.Pharmacoeconomics. 2015 May;33(5):445-55. doi: 10.1007/s40273-014-0251-x. Pharmacoeconomics. 2015. PMID: 25630758 Free PMC article. Review.
Cited by
-
Multimethod quantitative benefit-risk assessment of treatments for moderate-to-severe osteoarthritis.Br J Clin Pharmacol. 2022 Aug;88(8):3837-3846. doi: 10.1111/bcp.15309. Epub 2022 Apr 8. Br J Clin Pharmacol. 2022. PMID: 35277997 Free PMC article.
-
Personalizing Medical Treatment Decisions: Integrating Meta-analytic Treatment Comparisons with Patient-Specific Risks and Preferences.Med Decis Making. 2019 Nov;39(8):998-1009. doi: 10.1177/0272989X19884927. Epub 2019 Nov 9. Med Decis Making. 2019. PMID: 31707910 Free PMC article.
-
A novel measure of drug benefit-risk assessment based on Scale Loss Score.Stat Methods Med Res. 2019 Sep;28(9):2738-2753. doi: 10.1177/0962280218786526. Epub 2018 Jul 20. Stat Methods Med Res. 2019. PMID: 30025499 Free PMC article.
-
A comparison of various aggregation functions in multi-criteria decision analysis for drug benefit-risk assessment.Stat Methods Med Res. 2022 May;31(5):899-916. doi: 10.1177/09622802211072512. Epub 2022 Jan 19. Stat Methods Med Res. 2022. PMID: 35044274 Free PMC article.
-
Personalization of Medical Treatment Decisions: Simplifying Complex Models while Maintaining Patient Health Outcomes.Med Decis Making. 2022 May;42(4):450-460. doi: 10.1177/0272989X211037921. Epub 2021 Aug 20. Med Decis Making. 2022. PMID: 34416832 Free PMC article.
References
-
- Weernink MGM, Janus SIM, van Til JA, Raisch DW, van Manen JG, IJzerman MJ. A systematic review to identify the use of preference elicitation methods in healthcare decision making. Pharmaceut Med. 2014;28:175–85. - PubMed
-
- van Til JA, IJzerman MJ. Why should regulators consider using patient preferences in benefit-risk assessment? Pharmacoeconomics. 2013;32:1–4. - PubMed
-
- MDIC PCBR project group members. A framework for incorporating information of patient preferences regarding benefit and risk into regulatory assessments of new medical technology. Minneapolis: Medical Device Innovation Consortium; 2015.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

