The Salmonella Effector SteD Mediates MARCH8-Dependent Ubiquitination of MHC II Molecules and Inhibits T Cell Activation
- PMID: 27832589
- PMCID: PMC5104694
- DOI: 10.1016/j.chom.2016.10.007
The Salmonella Effector SteD Mediates MARCH8-Dependent Ubiquitination of MHC II Molecules and Inhibits T Cell Activation
Abstract
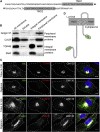
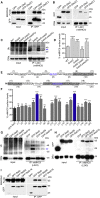
The SPI-2 type III secretion system (T3SS) of intracellular Salmonella enterica translocates effector proteins into mammalian cells. Infection of antigen-presenting cells results in SPI-2 T3SS-dependent ubiquitination and reduction of surface-localized mature MHC class II (mMHCII). We identify the effector SteD as required and sufficient for this process. In Mel Juso cells, SteD localized to the Golgi network and vesicles containing the E3 ubiquitin ligase MARCH8 and mMHCII. SteD caused MARCH8-dependent ubiquitination and depletion of surface mMHCII. One of two transmembrane domains and the C-terminal cytoplasmic region of SteD mediated binding to MARCH8 and mMHCII, respectively. Infection of dendritic cells resulted in SteD-dependent depletion of surface MHCII, the co-stimulatory molecule B7.2, and suppression of T cell activation. SteD also accounted for suppression of T cell activation during Salmonella infection of mice. We propose that SteD is an adaptor, forcing inappropriate ubiquitination of mMHCII by MARCH8 and thereby suppressing T cell activation.
Keywords: Salmonella; dendritic cells; effector; ligase; major histocompatibility complex; ubiquitin.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Salmonella Gives MARCH(ing) Orders to MHC-II.Cell Host Microbe. 2016 Nov 9;20(5):551-552. doi: 10.1016/j.chom.2016.10.019. Cell Host Microbe. 2016. PMID: 27832583
Similar articles
-
CD97 stabilises the immunological synapse between dendritic cells and T cells and is targeted for degradation by the Salmonella effector SteD.PLoS Pathog. 2021 Jul 27;17(7):e1009771. doi: 10.1371/journal.ppat.1009771. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34314469 Free PMC article.
-
The Tumour Suppressor TMEM127 Is a Nedd4-Family E3 Ligase Adaptor Required by Salmonella SteD to Ubiquitinate and Degrade MHC Class II Molecules.Cell Host Microbe. 2020 Jul 8;28(1):54-68.e7. doi: 10.1016/j.chom.2020.04.024. Epub 2020 Jun 10. Cell Host Microbe. 2020. PMID: 32526160 Free PMC article.
-
Salmonella escapes antigen presentation through K63 ubiquitination mediated endosomal proteolysis of MHC II via modulation of endosomal acidification in dendritic cells.Pathog Dis. 2018 Mar 1;76(2). doi: 10.1093/femspd/ftx125. Pathog Dis. 2018. PMID: 29293966
-
Molecular mechanism and cellular function of MHCII ubiquitination.Immunol Rev. 2015 Jul;266(1):134-44. doi: 10.1111/imr.12303. Immunol Rev. 2015. PMID: 26085212 Free PMC article. Review.
-
Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response.FEMS Immunol Med Microbiol. 2000 Apr;27(4):313-20. doi: 10.1111/j.1574-695X.2000.tb01445.x. FEMS Immunol Med Microbiol. 2000. PMID: 10727887 Review.
Cited by
-
CD97 stabilises the immunological synapse between dendritic cells and T cells and is targeted for degradation by the Salmonella effector SteD.PLoS Pathog. 2021 Jul 27;17(7):e1009771. doi: 10.1371/journal.ppat.1009771. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34314469 Free PMC article.
-
PRMT5 epigenetically regulates the E3 ubiquitin ligase ITCH to influence lipid accumulation during mycobacterial infection.PLoS Pathog. 2022 Jun 3;18(6):e1010095. doi: 10.1371/journal.ppat.1010095. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35658060 Free PMC article.
-
Interesting Biochemistries in the Structure and Function of Bacterial Effectors.Front Cell Infect Microbiol. 2021 Feb 24;11:608860. doi: 10.3389/fcimb.2021.608860. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33718265 Free PMC article. Review.
-
HPV upregulates MARCHF8 ubiquitin ligase and inhibits apoptosis by degrading the death receptors in head and neck cancer.PLoS Pathog. 2023 Mar 3;19(3):e1011171. doi: 10.1371/journal.ppat.1011171. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36867660 Free PMC article.
-
T cell expression of IL-18R and DR3 is essential for non-cognate stimulation of Th1 cells and optimal clearance of intracellular bacteria.PLoS Pathog. 2017 Aug 17;13(8):e1006566. doi: 10.1371/journal.ppat.1006566. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28817719 Free PMC article.
References
-
- Ashida H., Kim M., Sasakawa C. Exploitation of the host ubiquitin system by human bacterial pathogens. Nat. Rev. Microbiol. 2014;12:399–413. - PubMed
-
- Cheminay C., Möhlenbrink A., Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J. Immunol. 2005;174:2892–2899. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

