Defining the clinical pathway in cochrane diagnostic test accuracy reviews
- PMID: 27832765
- PMCID: PMC5103389
- DOI: 10.1186/s12874-016-0252-x
Defining the clinical pathway in cochrane diagnostic test accuracy reviews
Abstract
Background: The value of a medical test depends on the context in which it might be used. Ideally, questions, results and conclusions of a diagnostic test accuracy (DTA) systematic review should be presented in light of this context. There is increasing acceptance of the value for knowing the impact a test can have on downstream consequences such as costs, implications for further testing and treatment options however there is currently no explicit guidance on how to address this. Authors of a Cochrane diagnostic review have recently been asked to include the clinical pathway in which a test maybe used. We aimed to evaluate how authors were developing their clinical pathways in the light of this.
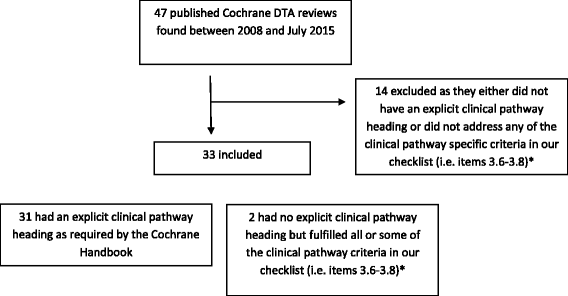
Methods: We searched the Cochrane Database of Systematic Reviews for all published DTA reviews. We included only those reviews that included a clinical pathway. We developed a checklist, based on the guidance in the Cochrane Handbook for DTA review authors. To this, we added a number of additional descriptors. We checked if the included pathways fulfilled these descriptors as defined by our checklist.
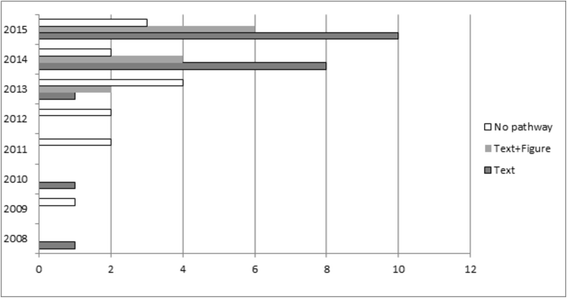
Results: We found 47 reviews, of which 33 (73 %) contained aspects pertaining to a clinical pathway. The 33 reviews addressed the clinical pathway differently, both in content and format. Of these, 21 provided a textual description and 12 include visual and textual descriptions. There was considerable variation in how comprehensively review authors adhered to our checklist. Eighteen reviews (51 %) linked the index test results to downstream clinical management actions and patient consequences, but only eight went on to differentially report on the consequences for false negative results and nine on the consequences for false positive results.
Conclusion: There is substantial variation in the clinical pathway descriptions in Cochrane systematic reviews of test accuracy. Most reviews do not link misclassifications (i.e. false negatives and false positive) to downstream patient consequences. Review authors could benefit from more explicit guidance on how to create such pathways, which in turn can help guide them in their evidence selection and appraisal of the evidence in the context of downstream consequences of testing.
Keywords: Clinical pathways; Cochrane systematic reviews; Diagnostic tests; Guidelines; Medical tests; Test accuracy studies.
Figures
Similar articles
-
How has the impact of 'care pathway technologies' on service integration in stroke care been measured and what is the strength of the evidence to support their effectiveness in this respect?Int J Evid Based Healthc. 2008 Mar;6(1):78-110. doi: 10.1111/j.1744-1609.2007.00098.x. Int J Evid Based Healthc. 2008. PMID: 21631815
-
Data sources and methods used to determine pretest probabilities in a cohort of Cochrane diagnostic test accuracy reviews.BMC Med Res Methodol. 2020 Apr 16;20(1):85. doi: 10.1186/s12874-020-00952-w. BMC Med Res Methodol. 2020. PMID: 32299367 Free PMC article.
-
Guidance was developed on how to write a plain language summary for diagnostic test accuracy reviews.J Clin Epidemiol. 2018 Nov;103:112-119. doi: 10.1016/j.jclinepi.2018.07.008. Epub 2018 Jul 21. J Clin Epidemiol. 2018. PMID: 30036677
-
lilacs search strategy for systematic reviews of diagnostic test accuracy studies.Health Info Libr J. 2019 Sep;36(3):223-243. doi: 10.1111/hir.12263. Epub 2019 Jul 4. Health Info Libr J. 2019. PMID: 31271504
-
Introduction to the Methods Guide for Medical Test Reviews.In: Chang SM, Matchar DB, Smetana GW, Umscheid CA, editors. Methods Guide for Medical Test Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Jun. Chapter 1. In: Chang SM, Matchar DB, Smetana GW, Umscheid CA, editors. Methods Guide for Medical Test Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Jun. Chapter 1. PMID: 22834022 Free Books & Documents. Review.
Cited by
-
Diagnostic accuracy of confocal microscopy imaging vs. punch biopsy for diagnosing and subtyping basal cell carcinoma.J Eur Acad Dermatol Venereol. 2017 Oct;31(10):1641-1648. doi: 10.1111/jdv.14253. Epub 2017 Aug 11. J Eur Acad Dermatol Venereol. 2017. PMID: 28370434 Free PMC article. Clinical Trial.
-
STARD 2015 was reproducible in a large set of studies on glaucoma.PLoS One. 2017 Oct 12;12(10):e0186209. doi: 10.1371/journal.pone.0186209. eCollection 2017. PLoS One. 2017. PMID: 29023557 Free PMC article.
-
Designing tailored maintenance strategies for systematic reviews and clinical practice guidelines using the Portfolio Maintenance by Test-Treatment (POMBYTT) framework.BMC Med Res Methodol. 2024 Feb 2;24(1):29. doi: 10.1186/s12874-024-02155-z. BMC Med Res Methodol. 2024. PMID: 38308228 Free PMC article. Review.
-
Accuracy of optical coherence tomography for diagnosing glaucoma: an overview of systematic reviews.Br J Ophthalmol. 2021 Apr;105(4):490-495. doi: 10.1136/bjophthalmol-2020-316152. Epub 2020 Jun 3. Br J Ophthalmol. 2021. PMID: 32493760 Free PMC article.
-
Update on the Role of Cytokines as Oral Biomarkers in the Diagnosis of Periodontitis.Adv Exp Med Biol. 2022;1373:283-302. doi: 10.1007/978-3-030-96881-6_15. Adv Exp Med Biol. 2022. PMID: 35612804
References
-
- Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106–10. doi: 10.1136/bmj.39500.677199.AE. - DOI - PMC - PubMed
-
- AHRQ . Methods Guide for Medical Test Reviews. AHRQ Publication No. 12-EC017. Rockville: Agency for Healthcare Research and Quality; 2012. - PubMed
-
- Deeks JJ, Wisniewski S, Davenport C. Chapter 4: Guide to the contents of a Cochrane Diagnostic Test Accuracy Protocol. In: Deeks JJ, Bossuyt P, Gatsonis C, editors. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy V100. Available from: http://srdta.cochrane.org/: Cochrane Collaboration; 2013.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

