In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model
- PMID: 27832815
- PMCID: PMC5103374
- DOI: 10.1186/s13287-016-0420-2
In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model
Abstract
Background: Human adipose-derived mesenchymal stem cells (haMSCs) have shown efficacy in treating osteoarthritis (OA) both preclinically and clinically via intra-articular (IA) injection. However, understanding the mode of action of the cell therapy has been limited by cell tracking capability and correlation between the pharmacokinetics of the injected cells and the intended pharmacodynamics effect. This study aims to explore methodology and to understand in vivo biodistribution of clinical-grade haMSCs labeled with fluorescent dye and injected into an immunocompetent OA rat model.
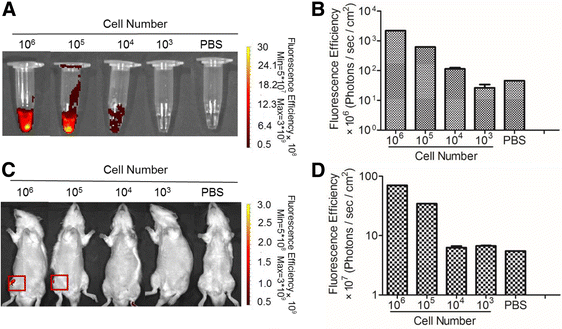
Methods: haMSCs labeled with fluorescent dye were investigated for their proliferation and differentiation capabilities. Labeled cells were used to establish detection threshold of a noninvasive biofluorescent imaging system before the cells (2.5 × 106) were injected into a conventional rat OA model induced by medial meniscectomy for 8 weeks. We attempted to reveal the existence of labeled cells in vivo by imaging and a molecular biomarker approach, and to correlate with the in vivo efficacy and physical presence over a follow-up period up to 10 weeks.
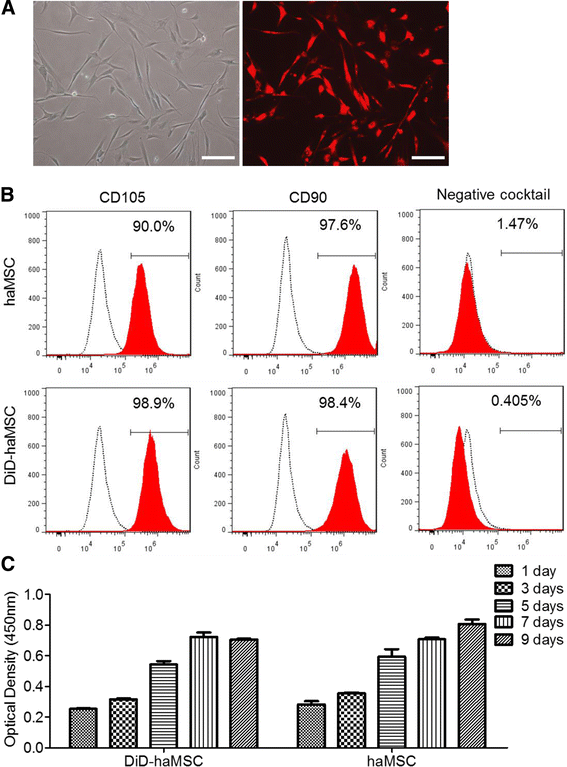
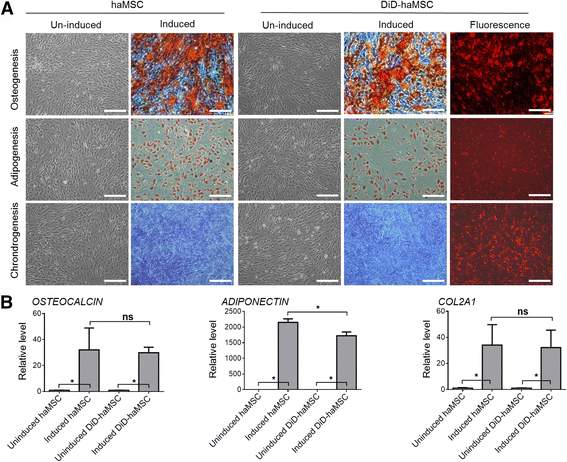
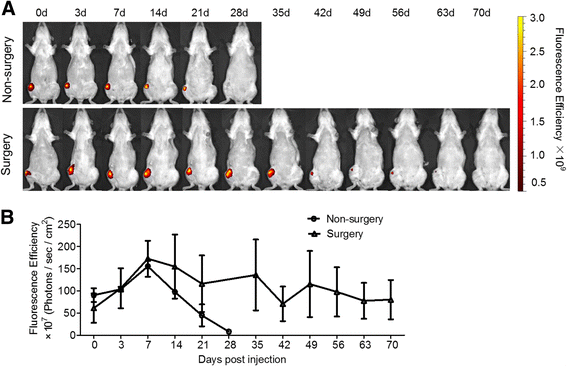
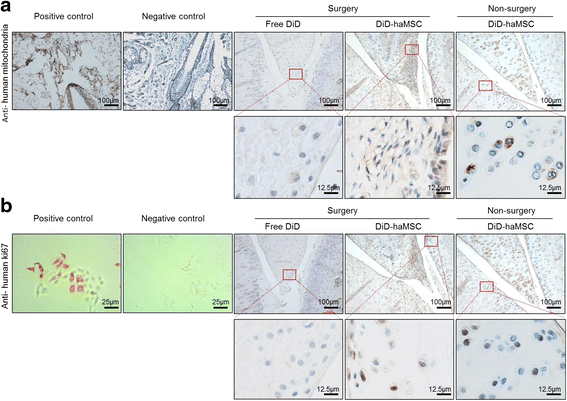
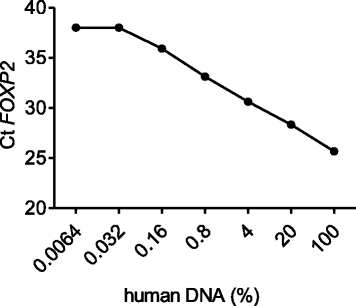
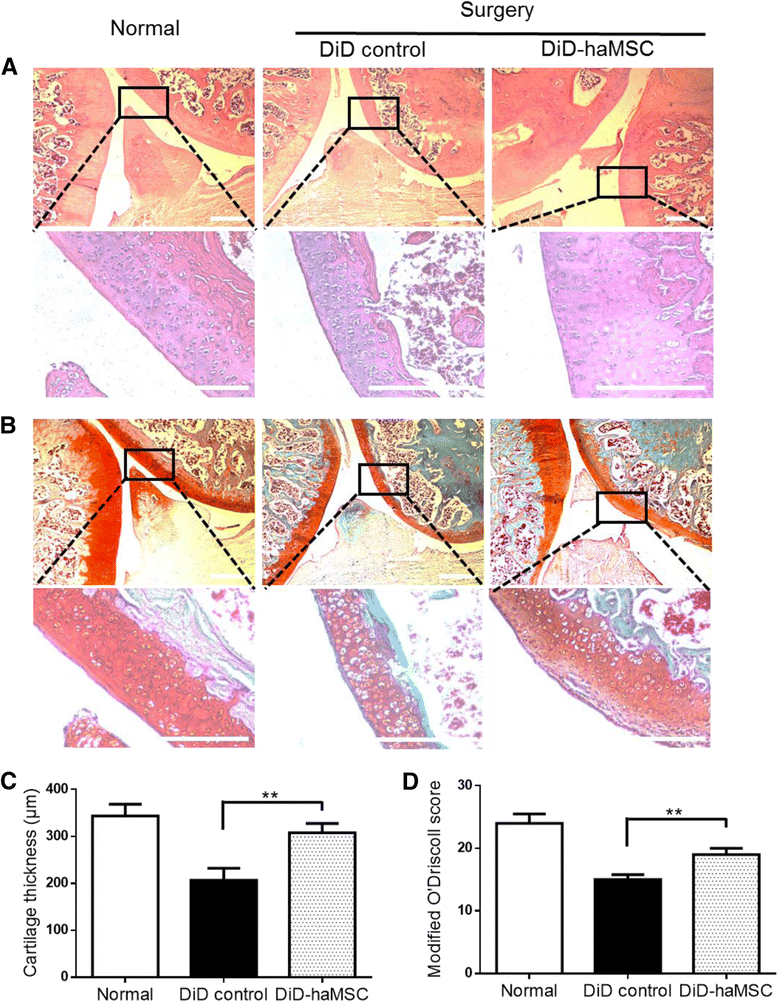
Results: In vitro proliferation and differentiation of haMSCs were not affected by the labeling of DiD dye. Detection thresholds of the labeled cells in vitro and in vivo were determined to be 104 and 105 cells, respectively. When 2.5 × 106 haMSCs were injected into the joints of a rat OA model, fluorescent signals (or >105 cells) lasted for about 10 weeks in the surgical knee joint at the same time as efficacy was observed. Signals in nonsurgical rats only lasted for 4 weeks. The human MSCs were shown to engraft to the rat joint tissues and were proliferative. Human FOXP2 gene was only detected in the knee joint tissue, suggesting limited biodistribution locally to the joints.
Conclusions: The current study represents the first attempt to correlate cell therapy efficacy on OA with the physical presence of the injected haMSCs in the OA model, and demonstrates that human adipose-derived mesenchymal stem cells persisted for 10 weeks locally in the rat joint, coinciding with the efficacy observed. It is postulated that persistence and/or proliferation of the haMSCs in the joint is required in order to exert their functions on promoting joint regeneration and/or cartilage protection, further supporting the safety and feasibility of IA injection of MSCs for the treatment of OA patients.
Keywords: Adipose-derived mesenchymal stem cells; Biodistribution; Carbocyanine dyes; Intra-articular injection; Osteoarthritis.
Figures
Similar articles
-
Efficacy and Persistence of Allogeneic Adipose-Derived Mesenchymal Stem Cells Combined with Hyaluronic Acid in Osteoarthritis After Intra-articular Injection in a Sheep Model.Tissue Eng Part A. 2018 Feb;24(3-4):219-233. doi: 10.1089/ten.TEA.2017.0039. Epub 2017 Sep 27. Tissue Eng Part A. 2018. PMID: 28486025
-
In Vivo Tracking of Human Adipose-derived Mesenchymal Stem Cells in a Rat Knee Osteoarthritis Model with Fluorescent Lipophilic Membrane Dye.J Vis Exp. 2017 Oct 8;(128):56273. doi: 10.3791/56273. J Vis Exp. 2017. PMID: 29053693 Free PMC article.
-
In vivo tracking and fate of intra-articularly injected superparamagnetic iron oxide particle-labeled multipotent stromal cells in an ovine model of osteoarthritis.Cell Transplant. 2015;24(11):2379-90. doi: 10.3727/096368914X685654. Epub 2014 Dec 12. Cell Transplant. 2015. PMID: 25506789
-
Treatment of osteoarthritis with mesenchymal stem cells.Sci China Life Sci. 2014 Jun;57(6):586-95. doi: 10.1007/s11427-014-4673-7. Epub 2014 May 21. Sci China Life Sci. 2014. PMID: 24849513 Review.
-
Tissue Engineering in Osteoarthritis: Current Status and Prospect of Mesenchymal Stem Cell Therapy.BioDrugs. 2018 Jun;32(3):183-192. doi: 10.1007/s40259-018-0276-3. BioDrugs. 2018. PMID: 29704190 Review.
Cited by
-
Alleviation of medial meniscal transection-induced osteoarthritis pain in rats by human adipose derived mesenchymal stem cells.Stem Cell Investig. 2020 Jun 17;7:10. doi: 10.21037/sci-2020-003. eCollection 2020. Stem Cell Investig. 2020. PMID: 32695803 Free PMC article.
-
The promise(s) of mesenchymal stem cell therapy in averting preclinical diabetes: lessons from in vivo and in vitro model systems.Sci Rep. 2021 Aug 20;11(1):16983. doi: 10.1038/s41598-021-96121-0. Sci Rep. 2021. PMID: 34417511 Free PMC article.
-
Research Progress on Stem Cell Therapies for Articular Cartilage Regeneration.Stem Cells Int. 2021 Feb 12;2021:8882505. doi: 10.1155/2021/8882505. eCollection 2021. Stem Cells Int. 2021. PMID: 33628274 Free PMC article. Review.
-
Human mesenchymal stem/stromal cell based-therapy in diabetes mellitus: experimental and clinical perspectives.Stem Cell Res Ther. 2024 Oct 29;15(1):384. doi: 10.1186/s13287-024-03974-z. Stem Cell Res Ther. 2024. PMID: 39468609 Free PMC article. Review.
-
Infrapatellar fat pad-derived MSC response to inflammation and fibrosis induces an immunomodulatory phenotype involving CD10-mediated Substance P degradation.Sci Rep. 2019 Jul 26;9(1):10864. doi: 10.1038/s41598-019-47391-2. Sci Rep. 2019. PMID: 31350444 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

