Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta
- PMID: 27832825
- PMCID: PMC5103372
- DOI: 10.1186/s13287-016-0418-9
Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta
Abstract
Background: Mesenchymal stem cells (MSCs) have been widely proven effective for therapeutic angiogenesis in ischemia animal models as well as clinical vascular diseases. Because of the invasive method, limited resources, and aging problems of adult tissue-derived MSCs, more perinatal tissue-derived MSCs have been isolated and studied as promising substitutable MSCs for cell transplantation. However, fewer studies have comparatively studied the angiogenic efficacy of MSCs derived from different tissues sources. Here, we evaluated whether the in-situ environment would affect the angiogenic potential of MSCs.
Methods: We harvested MSCs from adult bone marrow (BMSCs), adipose tissue (AMSCs), perinatal umbilical cord (UMSCs), and placental chorionic villi (PMSCs), and studied their "MSC identity" by flow cytometry and in-vitro trilineage differentiation assay. Then we comparatively studied their endothelial differentiation capabilities and paracrine actions side by side in vitro.
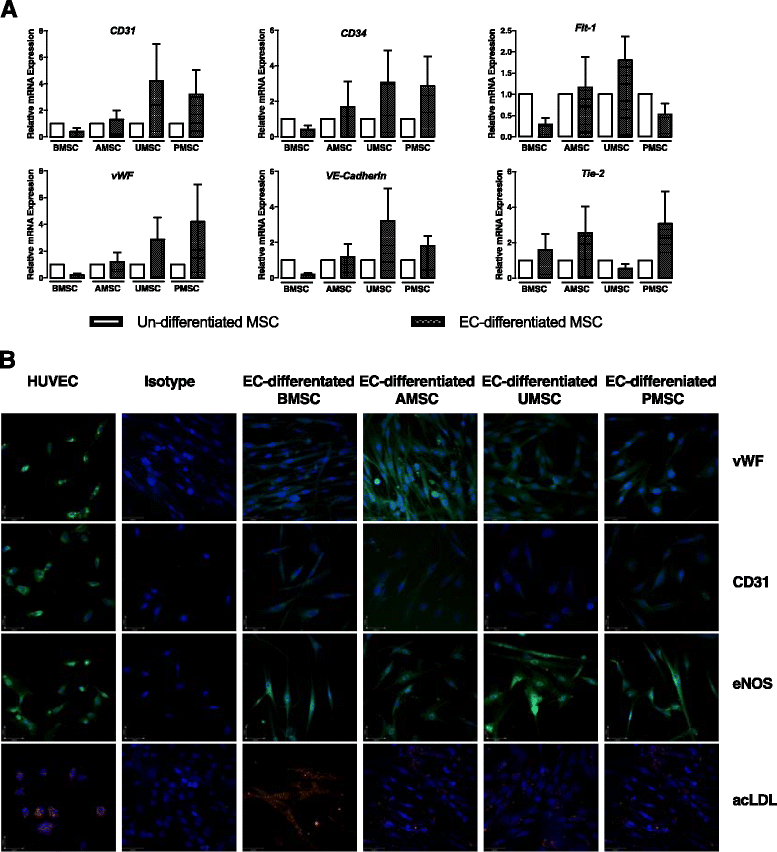
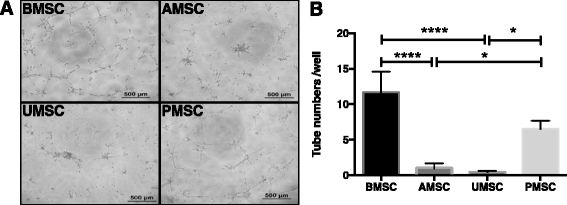
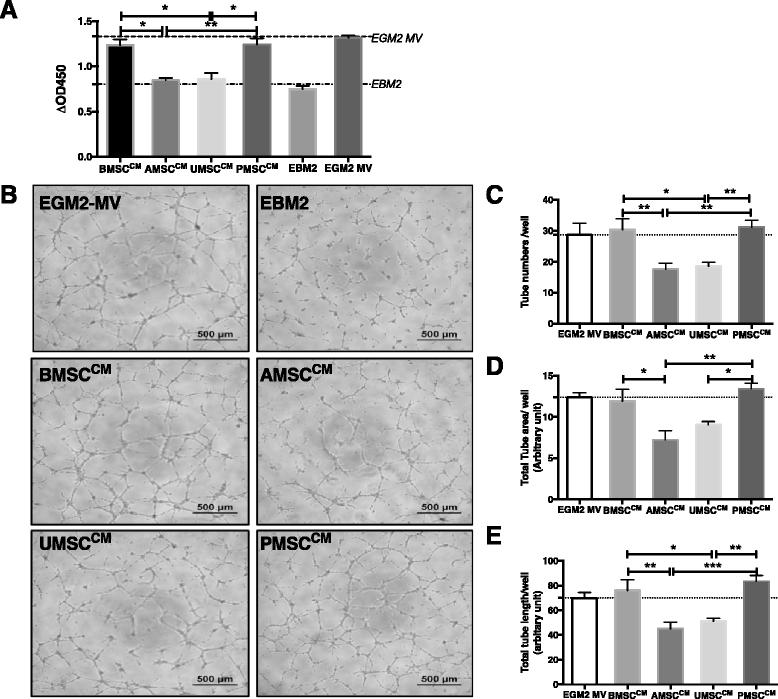
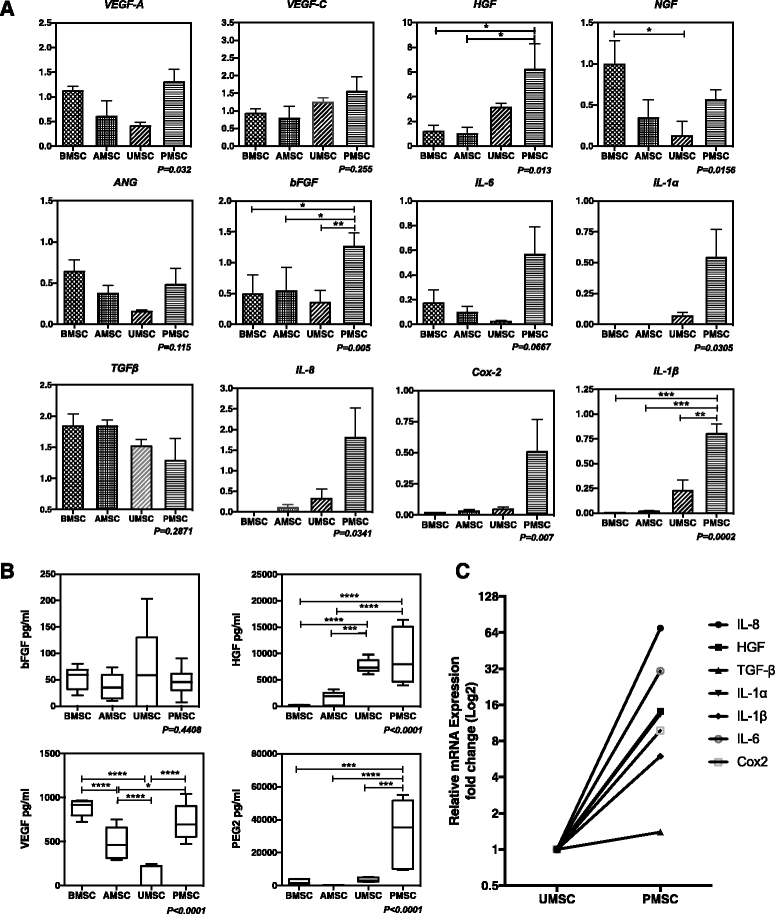
Results: Our data showed that UMSCs and PMSCs fitted well with the minimum standard of MSCs as well as BMSCs and AMSCs. Interestingly, we found that MSCs regardless of their tissue origins could develop similar endothelial-relevant functions in vitro, including producing eNOS and uptaking ac-LDL during endothelial differentiation in spite of their feeble expression of endothelial-related genes and proteins. Additionally, we surprisingly found that BMSCs and PMSCs could directly form tubular structures in vitro on Matrigel and their conditioned medium showed significant proangiogenic bioactivities on endothelial cells in vitro compared with those of AMSCs and UMSCs. Besides, several angiogenic genes were upregulated in BMSCs and PMSCs in comparison with AMSCs and UMSCs. Moreover, enzyme-linked immunosorbent assay further confirmed that BMSCs secreted much more VEGF, and PMSCs secreted much more HGF and PGE2.
Conclusions: Our study demonstrated the heterogeneous proangiogenic properties of MSCs derived from different tissue origins, and the in vivo isolated environment might contribute to these differences. Our study suggested that MSCs derived from bone marrow and placental chorionic villi might be preferred in clinical application for therapeutic angiogenesis.
Keywords: Adipose tissue; Bone marrow; Heterogeneity; Mesenchymal stem cells; Placental chorionic villi; Pro-angiogenic features; Umbilical cord.
Figures
Similar articles
-
New insights into the heterogeneity and functional diversity of human mesenchymal stem cells.Biomed Mater Eng. 2017;28(s1):S29-S45. doi: 10.3233/BME-171622. Biomed Mater Eng. 2017. PMID: 28372276
-
Comparison of human mesenchymal stem cells derived from dental pulp, bone marrow, adipose tissue, and umbilical cord tissue by gene expression.Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014 Sep;158(3):373-7. doi: 10.5507/bp.2013.078. Epub 2013 Oct 18. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014. PMID: 24145770
-
Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue.Int J Mol Med. 2016 Jan;37(1):115-25. doi: 10.3892/ijmm.2015.2413. Epub 2015 Nov 19. Int J Mol Med. 2016. PMID: 26719857 Free PMC article.
-
Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin.Am J Sports Med. 2019 Jun;47(7):1722-1733. doi: 10.1177/0363546519848678. Epub 2019 May 17. Am J Sports Med. 2019. PMID: 31100005 Review.
-
Umbilical cord-derived mesenchymal stromal cells in cardiovascular disease: review of preclinical and clinical data.Cytotherapy. 2019 Oct;21(10):1007-1018. doi: 10.1016/j.jcyt.2019.04.056. Epub 2019 Sep 17. Cytotherapy. 2019. PMID: 31540804 Review.
Cited by
-
Mesenchymal Stem Cells in Clinical Trials for Immune Disorders.Glob Med Genet. 2024 Jun 27;11(3):196-199. doi: 10.1055/s-0044-1788044. eCollection 2024 Sep. Glob Med Genet. 2024. PMID: 38947762 Free PMC article. No abstract available.
-
Aldehyde dehydrogenase activity of Wharton jelly mesenchymal stromal cells: isolation and characterization.Cytotechnology. 2019 Feb;71(1):427-441. doi: 10.1007/s10616-018-0283-8. Epub 2019 Jan 4. Cytotechnology. 2019. PMID: 30610510 Free PMC article.
-
Towards the Standardization of Mesenchymal Stem Cell Secretome-Derived Product Manufacturing for Tissue Regeneration.Int J Mol Sci. 2023 Aug 9;24(16):12594. doi: 10.3390/ijms241612594. Int J Mol Sci. 2023. PMID: 37628774 Free PMC article. Review.
-
Mesenchymal Stem Cells in Soft Tissue Regenerative Medicine: A Comprehensive Review.Medicina (Kaunas). 2023 Aug 10;59(8):1449. doi: 10.3390/medicina59081449. Medicina (Kaunas). 2023. PMID: 37629738 Free PMC article. Review.
-
In vitro and in ovo impact of the ionic dissolution products of boron-doped bioactive silicate glasses on cell viability, osteogenesis and angiogenesis.Sci Rep. 2022 May 20;12(1):8510. doi: 10.1038/s41598-022-12430-y. Sci Rep. 2022. PMID: 35595847 Free PMC article.
References
-
- Huang PP, Yang XF, Li SZ, Wen JC, Zhang Y, Han ZC. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb Haemost. 2007;98(6):1335–1342. - PubMed
-
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–274. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

