Essential medicines for universal health coverage
- PMID: 27832874
- PMCID: PMC7159295
- DOI: 10.1016/S0140-6736(16)31599-9
Essential medicines for universal health coverage
Figures
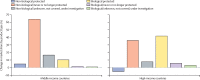
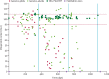
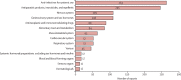

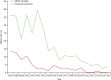
Comment in
-
Essential medicines for universal health coverage.Lancet. 2017 Jan 28;389(10067):337-339. doi: 10.1016/S0140-6736(16)31907-9. Epub 2016 Nov 8. Lancet. 2017. PMID: 27832878 No abstract available.
-
Veronika Wirtz: global leader in improving access to medicines.Lancet. 2017 Jan 28;389(10067):357. doi: 10.1016/S0140-6736(16)32137-7. Epub 2016 Nov 8. Lancet. 2017. PMID: 27836190 No abstract available.
-
Essential medicines for universal health coverage.Lancet. 2017 May 13;389(10082):1880-1881. doi: 10.1016/S0140-6736(17)31211-4. Epub 2017 May 11. Lancet. 2017. PMID: 28513444 No abstract available.
References
-
- UN Sustainable development goals. 2015. https://sustainabledevelopment.un.org/sdgs (accessed March 4, 2016).
-
- WHO What is universal health coverage? http://www.who.int/universal_health_coverage/en/index.html (accessed April 9, 2016).
-
- Wagner AK, Graves AJ, Reiss SK, Lecates R, Zhang F, Ross-Degnan D. Access to care and medicines, burden of health care expenditures, and risk protection: results from the World Health Survey. Health Policy. 2011;100:151–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

