Changes in empyema among U.S. children in the pneumococcal conjugate vaccine era
- PMID: 27832918
- PMCID: PMC5552045
- DOI: 10.1016/j.vaccine.2016.10.062
Changes in empyema among U.S. children in the pneumococcal conjugate vaccine era
Abstract
Background: Parapneumonic empyema, a serious complication of pneumonia, started increasing among U.S. children before the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) in 2000, and continued afterwards. This increase was due in part to pneumococcal serotypes not included in PCV7 that were included in the new 13-valent (PCV13) vaccine introduced in 2010. We assessed changes in the incidence of empyema hospitalizations among U.S. children after PCV13 introduction.
Methods: We calculated annualized empyema hospitalization rates among U.S. children <18years using Nationwide Inpatient Sample and Census data (1997-2013) for four periods based on PCV7 and PCV13 introductions. Relative rates (RR) and 95% confidence intervals (CI) were calculated by age group and sex, comparing PCV7 [early-PCV7 (2001-2005) and late-PCV7 (2006-2009)] and PCV13 (2011-2013) periods with the pre-PCV7 period (1997-1999). Secondary analyses examined changes in pneumococcal, streptococcal, staphylococcal and unspecified empyema.
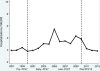
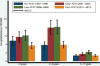
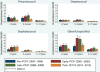
Results: Among children <18years of age, annualized empyema hospitalization rates peaked at 3.6 per 100,000 in the late-PCV7 period compared with 2.1 per 100,000 in the pre-PCV7 period [RR: 1.70 (95% CI: 1.11-2.60)]. However, annualized rates in the post-PCV13 period declined to 2.0 per 100,000, similar to rates in the pre-PCV7 period. Empyema rates among children <2years were lower in the post-PCV13 period compared to the pre-PCV7 period [RR: 0.77 (95% CI: 0.61-0.96)], but rates in the two periods among children 2-4 and 5-17years were similar. Most empyema were of unspecified etiology. Pneumococcal and unspecified empyema declined after PCV13 introduction.
Conclusions: Although empyema hospitalization rates among U.S. children peaked after PCV7 introduction, rates decreased substantially following the introduction of PCV13.
Keywords: Epidemiology; Invasive pneumococcal disease; Parapneumonic empyema; Pneumococcal conjugate vaccine.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

