Comparison of Biexponential and Monoexponential Model of Diffusion-Weighted Imaging for Distinguishing between Common Renal Cell Carcinoma and Fat Poor Angiomyolipoma
- PMID: 27833401
- PMCID: PMC5102913
- DOI: 10.3348/kjr.2016.17.6.853
Comparison of Biexponential and Monoexponential Model of Diffusion-Weighted Imaging for Distinguishing between Common Renal Cell Carcinoma and Fat Poor Angiomyolipoma
Abstract
Objective: To compare the diagnostic accuracy of intravoxel incoherent motion (IVIM)-derived parameters and apparent diffusion coefficient (ADC) in distinguishing between renal cell carcinoma (RCC) and fat poor angiomyolipoma (AML).
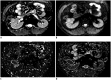
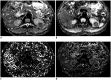
Materials and methods: Eighty-three patients with pathologically confirmed renal tumors were included in the study. All patients underwent renal 1.5T MRI, including IVIM protocol with 8 b values (0-800 s/mm2). The ADC, diffusion coefficient (D), pseudodiffusion coefficient (D*), and perfusion fraction (f) were calculated. One-way ANOVA was used for comparing ADC and IVIM-derived parameters among clear cell RCC (ccRCC), non-ccRCC and fat poor AML. The diagnostic performance of these parameters was evaluated by using receiver operating characteristic (ROC) analysis.
Results: The ADC were significantly greater in ccRCCs than that of non-ccRCCs and fat poor AMLs (each p < 0.010, respectively). The D and D* among the three groups were significantly different (all p < 0.050). The f of non-ccRCCs were less than that of ccRCCs and fat poor AMLs (each p < 0.050, respectively). In ROC analysis, ADC and D showed similar area under the ROC curve (AUC) values (AUC = 0.955 and 0.964, respectively, p = 0.589) in distinguishing between ccRCCs and fat poor AMLs. The combination of D > 0.97 × 10-3 mm2/s, D* < 28.03 × 10-3 mm2/s, and f < 13.61% maximized the diagnostic sensitivity for distinguishing non-ccRCCs from fat poor AMLs. The final estimates of AUC (95% confidence interval), sensitivity, specificity, positive predictive value, negative predictive value and accuracy for the entire cohort were 0.875 (0.719-0.962), 100% (23/23), 75% (9/12), 88.5% (23/26), 100% (9/9), and 91.4% (32/35), respectively.
Conclusion: The ADC and D showed similar diagnostic accuracy in distinguishing between ccRCCs and fat poor AMLs. The IVIM-derived parameters were better than ADC in discriminating non-ccRCCs from fat poor AMLs.
Keywords: Angiomyolipoma; DWI; Diffusion-weighted imaging; Intravoxel incoherent motion; Renal cell carcinoma.
Figures



Comment in
-
RE: Distinguishing between Renal Cell Carcinoma and Fat Poor Angiomyolipoma in Diffusion-Weighted Imaging.Korean J Radiol. 2017 Mar-Apr;18(2):410-411. doi: 10.3348/kjr.2017.18.2.410. Epub 2017 Feb 7. Korean J Radiol. 2017. PMID: 28246523 Free PMC article. No abstract available.
References
-
- Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–133. - PubMed
-
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. - PubMed
-
- Milner J, McNeil B, Alioto J, Proud K, Rubinas T, Picken M, et al. Fat poor renal angiomyolipoma: patient, computerized tomography and histological findings. J Urol. 2006;176:905–909. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

