cAMP Signaling Regulates Synchronised Growth of Symbiotic Epichloë Fungi with the Host Grass Lolium perenne
- PMID: 27833620
- PMCID: PMC5082231
- DOI: 10.3389/fpls.2016.01546
cAMP Signaling Regulates Synchronised Growth of Symbiotic Epichloë Fungi with the Host Grass Lolium perenne
Abstract
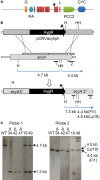
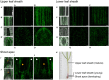
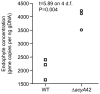
The seed-transmitted fungal symbiont, Epichloë festucae, colonizes grasses by infecting host tissues as they form on the shoot apical meristem (SAM) of the seedling. How this fungus accommodates the complexities of plant development to successfully colonize the leaves and inflorescences is unclear. Since adenosine 3', 5'-cyclic monophosphate (cAMP)-dependent signaling is often essential for host colonization by fungal pathogens, we disrupted the cAMP cascade by insertional mutagenesis of the E. festucae adenylate cyclase gene (acyA). Consistent with deletions of this gene in other fungi, acyA mutants had a slow radial growth rate in culture, and hyphae were convoluted and hyper-branched suggesting that fungal apical dominance had been disrupted. Nitro blue tetrazolium (NBT) staining of hyphae showed that cAMP disruption mutants were impaired in their ability to synthesize superoxide, indicating that cAMP signaling regulates accumulation of reactive oxygen species (ROS). Despite significant defects in hyphal growth and ROS production, E. festucae ΔacyA mutants were infectious and capable of forming symbiotic associations with grasses. Plants infected with E. festucae ΔacyA were marginally less robust than the wild-type (WT), however hyphae were hyper-branched, and leaf tissues heavily colonized, indicating that the tight regulation of hyphal growth normally observed in maturing leaves requires functional cAMP signaling.
Keywords: Epichloë festucae; Lolium perenne; cAMP signaling; hyphal branching; reactive oxygen species; symbiosis.
Figures





Similar articles
-
A homologue of the fungal tetraspanin Pls1 is required for Epichloë festucae expressorium formation and establishment of a mutualistic interaction with Lolium perenne.Mol Plant Pathol. 2019 Jul;20(7):961-975. doi: 10.1111/mpp.12805. Epub 2019 Apr 22. Mol Plant Pathol. 2019. PMID: 31008572 Free PMC article.
-
The Fungal Cell-Wall Integrity MAPK Cascade Is Crucial for Hyphal Network Formation and Maintenance of Restrictive Growth of Epichloë festucae in Symbiosis With Lolium perenne.Mol Plant Microbe Interact. 2015 Jan;28(1):69-85. doi: 10.1094/MPMI-06-14-0183-R. Mol Plant Microbe Interact. 2015. PMID: 25303335
-
SymB and SymC, two membrane associated proteins, are required for Epichloë festucae hyphal cell-cell fusion and maintenance of a mutualistic interaction with Lolium perenne.Mol Microbiol. 2017 Feb;103(4):657-677. doi: 10.1111/mmi.13580. Epub 2016 Dec 14. Mol Microbiol. 2017. PMID: 27882646
-
What triggers grass endophytes to switch from mutualism to pathogenism?Plant Sci. 2011 Feb;180(2):190-5. doi: 10.1016/j.plantsci.2010.10.002. Epub 2010 Oct 16. Plant Sci. 2011. PMID: 21421360 Review.
-
The fine balance between mutualism and antagonism in the Epichloë festucae-grass symbiotic interaction.Curr Opin Plant Biol. 2018 Aug;44:32-38. doi: 10.1016/j.pbi.2018.01.010. Epub 2018 Feb 16. Curr Opin Plant Biol. 2018. PMID: 29454183 Review.
Cited by
-
Transcriptome Analysis of Choke Stroma and Asymptomatic Inflorescence Tissues Reveals Changes in Gene Expression in Both Epichloë festucae and Its Host Plant Festuca rubra subsp. rubra.Microorganisms. 2019 Nov 16;7(11):567. doi: 10.3390/microorganisms7110567. Microorganisms. 2019. PMID: 31744076 Free PMC article.
-
Epichloë Fungal Endophytes-From a Biological Curiosity in Wild Grasses to an Essential Component of Resilient High Performing Ryegrass and Fescue Pastures.J Fungi (Basel). 2020 Nov 27;6(4):322. doi: 10.3390/jof6040322. J Fungi (Basel). 2020. PMID: 33261217 Free PMC article. Review.
-
Genetic and molecular basis of carotenoid metabolism in cereals.Theor Appl Genet. 2023 Mar 20;136(3):63. doi: 10.1007/s00122-023-04336-8. Theor Appl Genet. 2023. PMID: 36939900 Review.
-
Global Changes in Asexual Epichloë Transcriptomes during the Early Stages, from Seed to Seedling, of Symbiotum Establishment.Microorganisms. 2021 May 4;9(5):991. doi: 10.3390/microorganisms9050991. Microorganisms. 2021. PMID: 34064362 Free PMC article.
-
A homologue of the fungal tetraspanin Pls1 is required for Epichloë festucae expressorium formation and establishment of a mutualistic interaction with Lolium perenne.Mol Plant Pathol. 2019 Jul;20(7):961-975. doi: 10.1111/mpp.12805. Epub 2019 Apr 22. Mol Plant Pathol. 2019. PMID: 31008572 Free PMC article.
References
-
- Alspaugh J. A., Pukkila-Worley R., Harashima T., Cavallo L. M., Funnell D., Cox G. M., et al. (2002). Adenylyl cyclase functions downstream of the G alpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1, 75–84. 10.1128/EC.1.1.75-84.2002 - DOI - PMC - PubMed
-
- Arachevaleta M., Bacon C. W., Hoveland C. S., Radcliffe D. E. (1989). Effect of the tall fescue endophyte on plant response to environmental stress. Agron. J. 81, 83–90. 10.2134/agronj1989.00021962008100010015x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

