Lignin Composition and Structure Differs between Xylem, Phloem and Phellem in Quercus suber L
- PMID: 27833631
- PMCID: PMC5081372
- DOI: 10.3389/fpls.2016.01612
Lignin Composition and Structure Differs between Xylem, Phloem and Phellem in Quercus suber L
Abstract
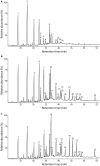
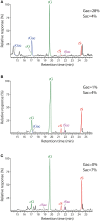
The composition and structure of lignin in different tissues-phellem (cork), phloem and xylem (wood)-of Quercus suber was studied. Whole cell walls and their respective isolated milled lignins were analyzed by pyrolysis coupled with gas chromatography/mass spectrometry (Py-GC/MS), two-dimensional nuclear magnetic resonance spectroscopy (2D-NMR) and derivatization followed by reductive cleavage (DFRC). Different tissues presented varied p-hydroxyphenyl:guaiacyl:syringyl (H:G:S) lignin compositions. Whereas lignin from cork has a G-rich lignin (H:G:S molar ratio 2:85:13), lignin from phloem presents more S-units (H:G:S molar ratio of 1:58:41) and lignin from xylem is slightly enriched in S-lignin (H:G:S molar ratio 1:45:55). These differences were reflected in the relative abundances of the different interunit linkages. Alkyl-aryl ethers (β-O-4') were predominant, increasing from 68% in cork, to 71% in phloem and 77% in xylem, as consequence of the enrichment in S-lignin units. Cork lignin was enriched in condensed structures such as phenylcoumarans (β-5', 20%), dibenzodioxocins (5-5', 5%), as corresponds to a lignin enriched in G-units. In comparison, lignin from phloem and xylem presented lower levels of condensed linkages. The lignin from cork was highly acetylated at the γ-OH of the side-chain (48% lignin acetylation), predominantly over G-units; while the lignins from phloem and xylem were barely acetylated and this occurred mainly over S-units. These results are a first time overview of the lignin structure in xylem, phloem (generated by cambium), and in cork (generated by phellogen), in agreement with literature that reports that lignin biosynthesis is flexible and cell specific.
Keywords: DFRC; NMR; Py-GC/MS; Quercus suber; cork; milled lignin; phloem; xylem.
Figures


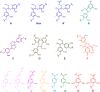
References
-
- Björkman A. (1956). Studies on finely divided wood. Part, I. Extraction of lignin with neutral solvents. Sven. Papperstidn. 13, 477–485.
-
- Boudet A. (2000). Lignins and lignification: selected issues. Plant Physiol. Biochem. 38, 81–96. 10.1016/S0981-9428(00)00166-2 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

