Extracellular Microvesicle Production by Human Eosinophils Activated by "Inflammatory" Stimuli
- PMID: 27833910
- PMCID: PMC5081571
- DOI: 10.3389/fcell.2016.00117
Extracellular Microvesicle Production by Human Eosinophils Activated by "Inflammatory" Stimuli
Abstract
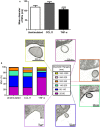
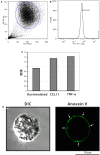
A key function of human eosinophils is to secrete cytokines, chemokines and cationic proteins, trafficking, and releasing these mediators for roles in inflammation and other immune responses. Eosinophil activation leads to secretion of pre-synthesized granule-stored mediators through different mechanisms, but the ability of eosinophils to secrete extracellular vesicles (EVs), very small vesicles with preserved membrane topology, is still poorly understood. In the present work, we sought to identify and characterize EVs released from human eosinophils during different conditions: after a culturing period or after isolation and stimulation with inflammatory stimuli, which are known to induce eosinophil activation and secretion: CCL11 (eotaxin-1) and tumor necrosis factor alpha (TNF-α). EV production was investigated by nanoscale flow cytometry, conventional transmission electron microscopy (TEM) and pre-embedding immunonanogold EM. The tetraspanins CD63 and CD9 were used as EV biomarkers for both flow cytometry and ultrastructural immunolabeling. Nanoscale flow cytometry showed that human eosinophils produce EVs in culture and that a population of EVs expressed detectable CD9, while CD63 was not consistently detected. When eosinophils were stimulated immediately after isolation and analyzed by TEM, EVs were clearly identified as microvesicles (MVs) outwardly budding off the plasma membrane. Both CCL11 and TNF-α induced significant increases of MVs compared to unstimulated cells. TNF-α induced amplified release of MVs more than CCL11. Eosinophil MV diameters varied from 20 to 1000 nm. Immunonanogold EM revealed clear immunolabeling for CD63 and CD9 on eosinophil MVs, although not all MVs were labeled. Altogether, we identified, for the first time, that human eosinophils secrete MVs and that this production increases in response to inflammatory stimuli. This is important to understand the complex secretory activities of eosinophils underlying immune responses. The contribution of the eosinophil-derived MVs to the regulation of immune responses awaits further investigation.
Keywords: CCL11 (eotaxin-1); CD63; CD9; cell secretion; inflammation; tetraspanins; transmission electron microscopy (TEM); tumor necrosis factor alpha (TNF-α).
Figures






Similar articles
-
CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils.J Leukoc Biol. 2016 Aug;100(2):391-401. doi: 10.1189/jlb.3A1015-480R. Epub 2016 Mar 10. J Leukoc Biol. 2016. PMID: 26965633 Free PMC article.
-
Single-Cell Analyses of Human Eosinophils at High Resolution to Understand Compartmentalization and Vesicular Trafficking of Interferon-Gamma.Front Immunol. 2018 Jul 9;9:1542. doi: 10.3389/fimmu.2018.01542. eCollection 2018. Front Immunol. 2018. PMID: 30038615 Free PMC article.
-
Expression and subcellular localization of the Qa-SNARE syntaxin17 in human eosinophils.Exp Cell Res. 2015 Oct 1;337(2):129-135. doi: 10.1016/j.yexcr.2015.07.003. Epub 2015 Aug 6. Exp Cell Res. 2015. PMID: 26254897 Free PMC article.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Vesicular trafficking of immune mediators in human eosinophils revealed by immunoelectron microscopy.Exp Cell Res. 2016 Oct 1;347(2):385-90. doi: 10.1016/j.yexcr.2016.08.016. Epub 2016 Aug 22. Exp Cell Res. 2016. PMID: 27562864 Free PMC article. Review.
Cited by
-
IL-33 stimulates the anticancer activities of eosinophils through extracellular vesicle-driven reprogramming of tumor cells.J Exp Clin Cancer Res. 2024 Jul 27;43(1):209. doi: 10.1186/s13046-024-03129-1. J Exp Clin Cancer Res. 2024. PMID: 39061080 Free PMC article.
-
Emerging Evidence for Pleiotropism of Eosinophils.Int J Mol Sci. 2021 Jun 30;22(13):7075. doi: 10.3390/ijms22137075. Int J Mol Sci. 2021. PMID: 34209213 Free PMC article. Review.
-
Proteomics of blood extracellular vesicles in inflammatory respiratory diseases for biomarker discovery and new insights into pathophysiology.Inflamm Regen. 2024 Sep 18;44(1):38. doi: 10.1186/s41232-024-00351-4. Inflamm Regen. 2024. PMID: 39294831 Free PMC article. Review.
-
Extracellular Vesicles (EVs) as Crucial Mediators of Cell-Cell Interaction in Asthma.Int J Mol Sci. 2023 Feb 28;24(5):4645. doi: 10.3390/ijms24054645. Int J Mol Sci. 2023. PMID: 36902079 Free PMC article. Review.
-
Contemporary understanding of the secretory granules in human eosinophils.J Leukoc Biol. 2018 Jul;104(1):85-93. doi: 10.1002/JLB.3MR1217-476R. Epub 2018 May 11. J Leukoc Biol. 2018. PMID: 29749658 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

