Development and Evaluation of a Duplex Real-Time PCR Assay With a Novel Internal Standard for Precise Quantification of Plasma DNA
- PMID: 27834061
- PMCID: PMC5107613
- DOI: 10.3343/alm.2017.37.1.18
Development and Evaluation of a Duplex Real-Time PCR Assay With a Novel Internal Standard for Precise Quantification of Plasma DNA
Abstract
Background: Circulating levels of cell-free DNA increase in many pathologic conditions. However, notable discrepancies in the quantitative analysis of cell-free DNA from a large number of laboratories have become a considerable pitfall, hampering its clinical application.
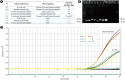
Methods: We designed a novel recombinant DNA fragment that could be applied as an internal standard in a newly developed and validated duplex real-time PCR assay for the quantitative analysis of total cell-free plasma DNA, which was tested in 5,442 healthy adults and 200 trauma patients.
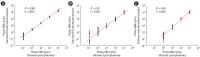
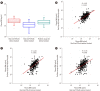
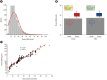
Results: Compared with two traditional methods, this novel assay showed a lower detection limit of 0.1 ng/mL, lower intra- and inter-assay CVs, and higher accuracy in the recovery test. The median plasma DNA concentration of healthy males (20.3 ng/mL, n=3,092) was significantly higher than that of healthy females (16.1 ng/mL, n=2,350) (Mann-Whitney two-sample rank sum test, P<0.0001). The reference intervals of plasma DNA concentration were 0-45.8 ng/mL and 0-52.5 ng/mL for healthy females and males, respectively. The plasma DNA concentrations of the majority of trauma patients (96%) were higher than the upper normal cutoff values and were closely related to the corresponding injury severity scores (R²=0.916, P<0.0001).
Conclusions: This duplex real-time PCR assay with a new internal standard could eliminate variation and allow for more sensitive, repeatable, accurate, and stable quantitative measurements of plasma DNA, showing promising application in clinical diagnosis.
Keywords: Duplex PCR; Internal standard; Plasma DNA; Quantification; Reference interval; Trauma.
Conflict of interest statement
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interests relevant to this article were reported.
Figures

Similar articles
-
Free circulating nucleic acids in plasma and serum as a novel approach to the use of internal controls in real time PCR based detection.J Virol Methods. 2014 Oct;207:133-7. doi: 10.1016/j.jviromet.2014.07.008. Epub 2014 Jul 14. J Virol Methods. 2014. PMID: 25034126
-
Development and inter-laboratory validation study of an improved new real-time PCR assay with internal control for detection and laboratory diagnosis of African swine fever virus.J Virol Methods. 2011 Dec;178(1-2):161-70. doi: 10.1016/j.jviromet.2011.09.007. Epub 2011 Sep 17. J Virol Methods. 2011. PMID: 21946285
-
A novel Alu-based real-time PCR method for the quantitative detection of plasma circulating cell-free DNA: sensitivity and specificity for the diagnosis of myocardial infarction.Int J Mol Med. 2015 Jan;35(1):72-80. doi: 10.3892/ijmm.2014.1991. Epub 2014 Nov 5. Int J Mol Med. 2015. PMID: 25374065 Free PMC article.
-
A duplex real-time PCR assay for the detection and quantification of avian reovirus and Mycoplasma synoviae.Virol J. 2015 Feb 12;12:22. doi: 10.1186/s12985-015-0255-y. Virol J. 2015. PMID: 25889592 Free PMC article.
-
[Quantitative PCR in the diagnosis of Leishmania].Parassitologia. 2004 Jun;46(1-2):163-7. Parassitologia. 2004. PMID: 15305709 Review. Italian.
Cited by
-
Clinical Characteristics of Severe COVID-19 Patients During Omicron Epidemic and a Nomogram Model Integrating Cell-Free DNA for Predicting Mortality: A Retrospective Analysis.Infect Drug Resist. 2023 Oct 18;16:6735-6745. doi: 10.2147/IDR.S430101. eCollection 2023. Infect Drug Resist. 2023. PMID: 37873032 Free PMC article.
-
Value of dynamic plasma cell-free DNA monitoring in septic shock syndrome: A case report.World J Clin Cases. 2020 Jan 6;8(1):200-207. doi: 10.12998/wjcc.v8.i1.200. World J Clin Cases. 2020. PMID: 31970188 Free PMC article.
-
Liquid biopsy for non-invasive assessment of liver injury in hepatitis B patients.World J Gastroenterol. 2019 Aug 7;25(29):3985-3995. doi: 10.3748/wjg.v25.i29.3985. World J Gastroenterol. 2019. PMID: 31413532 Free PMC article.
References
-
- Mandel P, Metais P. Nucleic acids in plasma samples from human blood. C R Seances Soc Biol Fil. 1948;142:241–243. - PubMed
-
- Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. - PubMed
-
- Tong YK, Lo YM. Diagnostic developments involving cell-free (circulating) nucleic acids. Clin Chim Acta. 2006;363:187–196. - PubMed
-
- Tissot C, Toffart AC, Villar S, Souquet PJ, Merle P, Moro-Sibilot D, et al. Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur Respir J. 2015;46:1773–1780. - PubMed
-
- Sozzi G, Roz L, Conte D, Mariani L, Andriani F, Lo Vullo S, et al. Plasma DNA quantification in lung cancer computed tomography screening: five-year results of a prospective study. Am J Respir Crit Care Med. 2009;179:69–74. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

