Results of the ICTuS 2 Trial (Intravascular Cooling in the Treatment of Stroke 2)
- PMID: 27834742
- PMCID: PMC5134910
- DOI: 10.1161/STROKEAHA.116.014200
Results of the ICTuS 2 Trial (Intravascular Cooling in the Treatment of Stroke 2)
Abstract
Background and purpose: Therapeutic hypothermia is a potent neuroprotectant approved for cerebral protection after neonatal hypoxia-ischemia and cardiac arrest. Therapeutic hypothermia for acute ischemic stroke is safe and feasible in pilot trials. We designed a study protocol to provide safer, faster therapeutic hypothermia in stroke patients.
Methods: Safety procedures and 4°C saline infusions for faster cooling were added to the ICTuS trial (Intravascular Cooling in the Treatment of Stroke) protocol. A femoral venous intravascular cooling catheter after intravenous recombinant tissue-type plasminogen activator in eligible patients provided 24 hours cooling followed by a 12-hour rewarm. Serial safety assessments and imaging were performed. The primary end point was 3-month modified Rankin score 0,1.
Results: Of the intended 1600 subjects, 120 were enrolled before the study was stopped. Randomly, 63 were to receive hypothermia plus antishivering treatment and 57 normothermia. Compared with previous studies, cooling rates were improved with a cold saline bolus, without fluid overload. The intention-to-treat primary outcome of 90-day modified Rankin Score 0,1 occurred in 33% hypothermia and 38% normothermia subjects, odds ratio (95% confidence interval) of 0.81 (0.36-1.85). Serious adverse events occurred equally. Mortality was 15.9% hypothermia and 8.8% normothermia subjects, odds ratio (95% confidence interval) of 1.95 (0.56-7.79). Pneumonia occurred in 19% hypothermia versus 10.5% in normothermia subjects, odds ratio (95% confidence interval) of 1.99 (0.63-6.98).
Conclusions: Intravascular therapeutic hypothermia was confirmed to be safe and feasible in recombinant tissue-type plasminogen activator-treated acute ischemic stroke patients. Protocol changes designed to reduce pneumonia risk appeared to fail, although the sample is small.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01123161.
Keywords: hypothermia; intention to treat analysis; ischemic stroke; neuroprotection; pneumonia; stroke; therapeutics.
© 2016 American Heart Association, Inc.
Figures

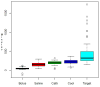

References
-
- Wu TC, Grotta JC. Hypothermia for acute ischaemic stroke. Lancet Neurol. 2013;12:275–284. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. - PubMed
-
- Bernard SG, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. New Engl J Med. 2002;346:557. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

