Tonsil-Derived Mesenchymal Stem Cells Differentiate into a Schwann Cell Phenotype and Promote Peripheral Nerve Regeneration
- PMID: 27834852
- PMCID: PMC5133867
- DOI: 10.3390/ijms17111867
Tonsil-Derived Mesenchymal Stem Cells Differentiate into a Schwann Cell Phenotype and Promote Peripheral Nerve Regeneration
Abstract
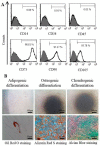
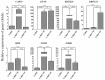
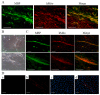
Schwann cells (SCs), which produce neurotropic factors and adhesive molecules, have been reported previously to contribute to structural support and guidance during axonal regeneration; therefore, they are potentially a crucial target in the restoration of injured nervous tissues. Autologous SC transplantation has been performed and has shown promising clinical results for treating nerve injuries and donor site morbidity, and insufficient production of the cells have been considered as a major issue. Here, we performed differentiation of tonsil-derived mesenchymal stem cells (T-MSCs) into SC-like cells (T-MSC-SCs), to evaluate T-MSC-SCs as an alternative to SCs. Using SC markers such as CAD19, GFAP, MBP, NGFR, S100B, and KROX20 during quantitative real-time PCR we detected the upregulation of NGFR, S100B, and KROX20 and the downregulation of CAD19 and MBP at the fully differentiated stage. Furthermore, we found myelination of axons when differentiated SCs were cocultured with mouse dorsal root ganglion neurons. The application of T-MSC-SCs to a mouse model of sciatic nerve injury produced marked improvements in gait and promoted regeneration of damaged nerves. Thus, the transplantation of human T-MSCs might be suitable for assisting in peripheral nerve regeneration.
Keywords: Schwann cell; differentiation; peripheral nerve; regeneration; tonsil-derived mesenchymal stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells into Schwann-Like Cells Improves Neuromuscular Function in a Mouse Model of Charcot-Marie-Tooth Disease Type 1A.Int J Mol Sci. 2018 Aug 14;19(8):2393. doi: 10.3390/ijms19082393. Int J Mol Sci. 2018. PMID: 30110925 Free PMC article.
-
KLF7-transfected Schwann cell graft transplantation promotes sciatic nerve regeneration.Neuroscience. 2017 Jan 6;340:319-332. doi: 10.1016/j.neuroscience.2016.10.069. Epub 2016 Nov 5. Neuroscience. 2017. PMID: 27826105
-
Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration.J Neuropathol Exp Neurol. 2010 Sep;69(9):973-85. doi: 10.1097/NEN.0b013e3181eff6dc. J Neuropathol Exp Neurol. 2010. PMID: 20720501
-
Transplantation of autologous Schwann cells for the repair of segmental peripheral nerve defects.Neurosurg Focus. 2009 Feb;26(2):E4. doi: 10.3171/FOC.2009.26.2.E4. Neurosurg Focus. 2009. PMID: 19435444 Review.
-
Schwann Cell Role in Selectivity of Nerve Regeneration.Cells. 2020 Sep 20;9(9):2131. doi: 10.3390/cells9092131. Cells. 2020. PMID: 32962230 Free PMC article. Review.
Cited by
-
Application of Mesenchymal Stem Cell-Derived Schwann Cell-like Cells Spared Neuromuscular Junctions and Enhanced Functional Recovery After Peripheral Nerve Injury.Cells. 2024 Dec 23;13(24):2137. doi: 10.3390/cells13242137. Cells. 2024. PMID: 39768225 Free PMC article.
-
Mesenchymal Stem Cells Expressing Baculovirus-Engineered Brain-Derived Neurotrophic Factor Improve Peripheral Nerve Regeneration in a Rat Model.Tissue Eng Regen Med. 2025 Apr;22(3):351-362. doi: 10.1007/s13770-025-00703-2. Epub 2025 Feb 17. Tissue Eng Regen Med. 2025. PMID: 39962026
-
Neural grafts containing exosomes derived from Schwann cell-like cells promote peripheral nerve regeneration in rats.Burns Trauma. 2023 Apr 27;11:tkad013. doi: 10.1093/burnst/tkad013. eCollection 2023. Burns Trauma. 2023. PMID: 37122841 Free PMC article.
-
Mesenchymal Stem Cells Derived from Wharton's Jelly Can Differentiate into Schwann Cell-Like Cells and Promote Peripheral Nerve Regeneration in Acellular Nerve Grafts.Tissue Eng Regen Med. 2021 Jun;18(3):467-478. doi: 10.1007/s13770-020-00329-6. Epub 2021 Jan 30. Tissue Eng Regen Med. 2021. PMID: 33515168 Free PMC article.
-
Transplantation of Differentiated Tonsil-Derived Mesenchymal Stem Cells Ameliorates Murine Duchenne Muscular Dystrophy via Autophagy Activation.Tissue Eng Regen Med. 2022 Dec;19(6):1283-1294. doi: 10.1007/s13770-022-00489-7. Epub 2022 Nov 1. Tissue Eng Regen Med. 2022. PMID: 36318366 Free PMC article.
References
-
- Lee Y.C., Chang M.H., Lin K.P. Charcot-Marie-Tooth disease. Acta Neurol. Taiwan. 2008;17:203–213. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

