PIN1 in breast development and cancer: a clinical perspective
- PMID: 27834957
- PMCID: PMC5299711
- DOI: 10.1038/cdd.2016.122
PIN1 in breast development and cancer: a clinical perspective
Abstract
Mammary gland development, various stages of mammary tumorigenesis and breast cancer progression have the peptidyl-prolyl cis/trans isomerase PIN1 at their centerpiece, in virtue of the ability of this unique enzyme to fine-tune the dynamic crosstalk between multiple molecular pathways. PIN1 exerts its action by inducing conformational and functional changes on key cellular proteins, following proline-directed phosphorylation. Through this post-phosphorylation signal transduction mechanism, PIN1 controls the extent and direction of the cellular response to a variety of inputs, in physiology and disease. This review discusses PIN1's roles in normal mammary development and cancerous progression, as well as the clinical impact of targeting this enzyme in breast cancer patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


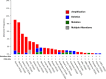
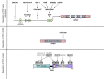
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. - PubMed
-
- Zhou ZZ. The isomerase PIN1 controls numerous cancer-driving pathways and is a unique drug target. Nat Rev Cancer 2016; 16: 463–478. - PubMed
-
- Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio F, Volinia S et al. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 2002; 419: 853–857. - PubMed
-
- Mantovani F, Piazza S, Gostissa M, Strano S, Zacchi P, Mantovani R et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol Cell 2004; 14: 625–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

