Stimulus-dependent dissociation between XB130 and Tks5 scaffold proteins promotes airway epithelial cell migration
- PMID: 27835612
- PMCID: PMC5363521
- DOI: 10.18632/oncotarget.13261
Stimulus-dependent dissociation between XB130 and Tks5 scaffold proteins promotes airway epithelial cell migration
Abstract
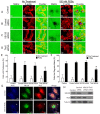
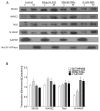
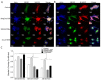
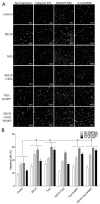
Repair of airway epithelium after injury requires migration of neighboring epithelial cells to injured areas. However, the molecular mechanisms regulating airway epithelial cell migration is not well defined. We have previously shown that XB130, a scaffold protein, is required for airway epithelial repair and regeneration in vivo, and interaction between XB130 and another scaffold protein, Tks5, regulates cell proliferation and survival in human bronchial epithelial cells. The objective of the present study was to determine the role of XB130 and Tks5 interaction in airway epithelial cell migration. Interestingly, we found that XB130 only promotes lateral cell migration, whereas, Tks5 promotes cell migration/invasion via proteolysis of extracellular matrix. Upon stimulation with EGF, PKC activator phorbol 12, 13-dibutyrate or a nicotinic acetylcholine receptor ligand, XB130 and Tks5 translocated to the cell membrane in a stimulus-dependent manner. The translocation and distribution of XB130 is similar to lamellipodial marker, WAVE2; whereas Tks5 is similar to podosome marker, N-WASP. Over-expression of XB130 or Tks5 alone enhances cell migration, whereas co-expression of both XB130 and Tks5 inhibits cell migration processes and signaling. Furthermore, XB130 interacts with Rac1 whereas Tks5 interacts with Cdc42 to promote Rho GTPase activity. Our results suggest that dissociation between XB130 and Tks5 may facilitate lateral cell migration via XB130/Rac1, and vertical cell migration via Tks5/Cdc42. These molecular mechanisms will help our understanding of airway epithelial repair and regeneration.
Keywords: Pathology Section; SH3PXD2A; actin filament associate protein 1-like 2; lamellipodia; lung repair; podosomes.
Conflict of interest statement
There is no conflict of interest.
Figures




References
-
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

