Azadiradione ameliorates polyglutamine expansion disease in Drosophila by potentiating DNA binding activity of heat shock factor 1
- PMID: 27835876
- PMCID: PMC5346638
- DOI: 10.18632/oncotarget.12930
Azadiradione ameliorates polyglutamine expansion disease in Drosophila by potentiating DNA binding activity of heat shock factor 1
Abstract
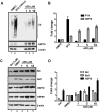
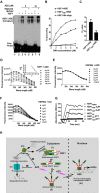
Aggregation of proteins with the expansion of polyglutamine tracts in the brain underlies progressive genetic neurodegenerative diseases (NDs) like Huntington's disease and spinocerebellar ataxias (SCA). An insensitive cellular proteotoxic stress response to non-native protein oligomers is common in such conditions. Indeed, upregulation of heat shock factor 1 (HSF1) function and its target protein chaperone expression has shown promising results in animal models of NDs. Using an HSF1 sensitive cell based reporter screening, we have isolated azadiradione (AZD) from the methanolic extract of seeds of Azadirachta indica, a plant known for its multifarious medicinal properties. We show that AZD ameliorates toxicity due to protein aggregation in cell and fly models of polyglutamine expansion diseases to a great extent. All these effects are correlated with activation of HSF1 function and expression of its target protein chaperone genes. Notably, HSF1 activation by AZD is independent of cellular HSP90 or proteasome function. Furthermore, we show that AZD directly interacts with purified human HSF1 with high specificity, and facilitates binding of HSF1 to its recognition sequence with higher affinity. These unique findings qualify AZD as an ideal lead molecule for consideration for drug development against NDs that affect millions worldwide.
Keywords: Gerotarget; HSF1; azadiradione; heat shock factor 1; neurodegenerative diseases; small molecule.
Conflict of interest statement
Authors declare no conflict of interests
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

