Lung Disease in Primary Antibody Deficiencies
- PMID: 27836055
- PMCID: PMC5129846
- DOI: 10.1016/j.jaip.2016.08.005
Lung Disease in Primary Antibody Deficiencies
Abstract
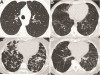
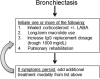
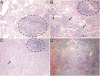
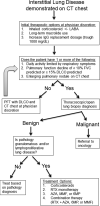
Primary antibody deficiencies (PADs) are the most common form of primary immunodeficiency and predispose to severe and recurrent pulmonary infections, which can result in chronic lung disease including bronchiectasis. Chronic lung disease is among the most common complications of PAD and a significant source of morbidity and mortality for these patients. However, the development of lung disease in PAD may not be solely the result of recurrent bacterial infection or a consequence of bronchiectasis. Recent characterization of monogenic immune dysregulation disorders and more extensive study of common variable immunodeficiency have demonstrated that interstitial lung disease (ILD) in PAD can result from generalized immune dysregulation and frequently occurs in the absence of pneumonia history or bronchiectasis. This distinction between bronchiectasis and ILD has important consequences in the evaluation and management of lung disease in PAD. For example, treatment of ILD in PAD typically uses immunomodulatory approaches in addition to immunoglobulin replacement and antibiotic prophylaxis, which are the stalwarts of bronchiectasis management in these patients. Although all antibody-deficient patients are at risk of developing bronchiectasis, ILD occurs in some forms of PAD much more commonly than in others, suggesting that distinct but poorly understood immunological factors underlie the development of this complication. Importantly, ILD can have earlier onset and may worsen survival more than bronchiectasis. Further efforts to understand the pathogenesis of lung disease in PAD will provide vital information for the most effective methods of diagnosis, surveillance, and treatment of these patients.
Keywords: Bronchiectasis; CVID; Common variable immunodeficiency; Granulomatous interstitial lung disease; Interstitial lung disease; Primary antibody deficiency.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

