Lethal H1N1 influenza A virus infection alters the murine alveolar type II cell surfactant lipidome
- PMID: 27836900
- PMCID: PMC5206402
- DOI: 10.1152/ajplung.00339.2016
Lethal H1N1 influenza A virus infection alters the murine alveolar type II cell surfactant lipidome
Abstract
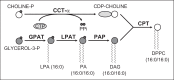
Alveolar type II (ATII) epithelial cells are the primary site of influenza virus replication in the distal lung. Development of acute respiratory distress syndrome in influenza-infected mice correlates with significant alterations in ATII cell function. However, the impact of infection on ATII cell surfactant lipid metabolism has not been explored. C57BL/6 mice were inoculated intranasally with influenza A/WSN/33 (H1N1) virus (10,000 plaque-forming units/mouse) or mock-infected with virus diluent. ATII cells were isolated by a standard lung digestion protocol at 2 and 6 days postinfection. Levels of 77 surfactant lipid-related compounds of known identity in each ATII cell sample were measured by ultra-high-performance liquid chromatography-mass spectrometry. In other mice, bronchoalveolar lavage fluid was collected to measure lipid and protein content using commercial assay kits. Relative to mock-infected animals, ATII cells from influenza-infected mice contained reduced levels of major surfactant phospholipids (phosphatidylcholine, phosphatidylglycerol, and phosphatidylethanolamine) but increased levels of minor phospholipids (phosphatidylserine, phosphatidylinositol, and sphingomyelin), cholesterol, and diacylglycerol. These changes were accompanied by reductions in cytidine 5'-diphosphocholine and 5'-diphosphoethanolamine (liponucleotide precursors for ATII cell phosphatidylcholine and phosphatidylethanolamine synthesis, respectively). ATII cell lamellar bodies were ultrastructurally abnormal after infection. Changes in ATII cell phospholipids were reflected in the composition of bronchoalveolar lavage fluid, which contained reduced amounts of phosphatidylcholine and phosphatidylglycerol but increased amounts of sphingomyelin, cholesterol, and protein. Influenza infection significantly alters ATII cell surfactant lipid metabolism, which may contribute to surfactant dysfunction and development of acute respiratory distress syndrome in influenza-infected mice.
Keywords: acute respiratory distress syndrome; alveolar type II cell; influenza; lipidomics; liponucleotide; mouse; phospholipid; surfactant.
Copyright © 2016 the American Physiological Society.
Figures





Similar articles
-
Postexposure Liponucleotide Prophylaxis and Treatment Attenuates Acute Respiratory Distress Syndrome in Influenza-infected Mice.Am J Respir Cell Mol Biol. 2021 Jun;64(6):677-686. doi: 10.1165/rcmb.2020-0465OC. Am J Respir Cell Mol Biol. 2021. PMID: 33606602 Free PMC article.
-
Infection of mice with influenza A/WSN/33 (H1N1) virus alters alveolar type II cell phenotype.Am J Physiol Lung Cell Mol Physiol. 2015 Apr 1;308(7):L628-38. doi: 10.1152/ajplung.00373.2014. Epub 2015 Jan 16. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25595651 Free PMC article.
-
Cytidine 5'-Diphosphocholine Corrects Alveolar Type II Cell Mitochondrial Dysfunction in Influenza-infected Mice.Am J Respir Cell Mol Biol. 2022 Jun;66(6):682-693. doi: 10.1165/rcmb.2021-0512OC. Am J Respir Cell Mol Biol. 2022. PMID: 35442170 Free PMC article.
-
Regulation of lung surfactant phospholipid synthesis and metabolism.Biochim Biophys Acta. 2013 Feb;1831(2):448-58. doi: 10.1016/j.bbalip.2012.11.009. Epub 2012 Nov 27. Biochim Biophys Acta. 2013. PMID: 23200861 Review.
-
Surfactant alteration and replacement in acute respiratory distress syndrome.Respir Res. 2001;2(6):353-64. doi: 10.1186/rr86. Epub 2001 Oct 12. Respir Res. 2001. PMID: 11737935 Free PMC article. Review.
Cited by
-
Advances and Applications of Lung Organoids in the Research on Acute Respiratory Distress Syndrome (ARDS).J Clin Med. 2024 Jan 8;13(2):346. doi: 10.3390/jcm13020346. J Clin Med. 2024. PMID: 38256480 Free PMC article. Review.
-
Metabolic shifts modulate lung injury caused by infection with H1N1 influenza A virus.Virology. 2021 Jul;559:111-119. doi: 10.1016/j.virol.2021.03.008. Epub 2021 Apr 6. Virology. 2021. PMID: 33865074 Free PMC article.
-
The inducible amphisome isolates viral hemagglutinin and defends against influenza A virus infection.Nat Commun. 2020 Jan 9;11(1):162. doi: 10.1038/s41467-019-13974-w. Nat Commun. 2020. PMID: 31919357 Free PMC article.
-
HIF-1α induces glycolytic reprograming in tissue-resident alveolar macrophages to promote cell survival during acute lung injury.Elife. 2022 Jul 13;11:e77457. doi: 10.7554/eLife.77457. Elife. 2022. PMID: 35822617 Free PMC article.
-
Association between lipid profiles and viral respiratory infections in human sputum samples.Respir Res. 2022 Jul 2;23(1):177. doi: 10.1186/s12931-022-02091-w. Respir Res. 2022. PMID: 35780155 Free PMC article.
References
-
- Aeffner F, Traylor ZP, Yu EN, Davis IC. Double-stranded RNA induces similar pulmonary dysfunction to respiratory syncytial virus in BALB/c mice. Am J Physiol Lung Cell Mol Physiol 301: L99–L109, 2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

