Spatial model of convective solute transport in brain extracellular space does not support a "glymphatic" mechanism
- PMID: 27836940
- PMCID: PMC5129742
- DOI: 10.1085/jgp.201611684
Spatial model of convective solute transport in brain extracellular space does not support a "glymphatic" mechanism
Abstract
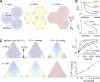
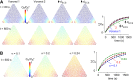
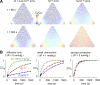
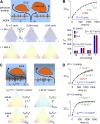
A "glymphatic system," which involves convective fluid transport from para-arterial to paravenous cerebrospinal fluid through brain extracellular space (ECS), has been proposed to account for solute clearance in brain, and aquaporin-4 water channels in astrocyte endfeet may have a role in this process. Here, we investigate the major predictions of the glymphatic mechanism by modeling diffusive and convective transport in brain ECS and by solving the Navier-Stokes and convection-diffusion equations, using realistic ECS geometry for short-range transport between para-arterial and paravenous spaces. Major model parameters include para-arterial and paravenous pressures, ECS volume fraction, solute diffusion coefficient, and astrocyte foot-process water permeability. The model predicts solute accumulation and clearance from the ECS after a step change in solute concentration in para-arterial fluid. The principal and robust conclusions of the model are as follows: (a) significant convective transport requires a sustained pressure difference of several mmHg between the para-arterial and paravenous fluid and is not affected by pulsatile pressure fluctuations; (b) astrocyte endfoot water permeability does not substantially alter the rate of convective transport in ECS as the resistance to flow across endfeet is far greater than in the gaps surrounding them; and (c) diffusion (without convection) in the ECS is adequate to account for experimental transport studies in brain parenchyma. Therefore, our modeling results do not support a physiologically important role for local parenchymal convective flow in solute transport through brain ECS.
© 2016 Jin et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

