Correlates of tuberculosis risk: predictive biomarkers for progression to active tuberculosis
- PMID: 27836953
- PMCID: PMC5898936
- DOI: 10.1183/13993003.01012-2016
Correlates of tuberculosis risk: predictive biomarkers for progression to active tuberculosis
Abstract
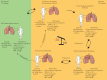
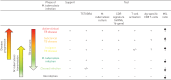
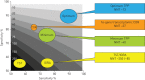
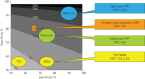
New approaches to control the spread of tuberculosis (TB) are needed, including tools to predict development of active TB from latent TB infection (LTBI). Recent studies have described potential correlates of risk, in order to inform the development of prognostic tests for TB disease progression. These efforts have included unbiased approaches employing "omics" technologies, as well as more directed, hypothesis-driven approaches assessing a small set or even individual selected markers as candidate correlates of TB risk. Unbiased high-throughput screening of blood RNAseq profiles identified signatures of active TB risk in individuals with LTBI, ≥1 year before diagnosis. A recent infant vaccination study identified enhanced expression of T-cell activation markers as a correlate of risk prior to developing TB; conversely, high levels of Ag85A antibodies and high frequencies of interferon (IFN)-γ specific T-cells were associated with reduced risk of disease. Others have described CD27-IFN-γ+CD4+ T-cells as possibly predictive markers of TB disease. T-cell responses to TB latency antigens, including heparin-binding haemagglutinin and DosR-regulon-encoded antigens have also been correlated with protection.Further studies are needed to determine whether correlates of risk can be used to prevent active TB through targeted prophylactic treatment, or to allow targeted enrolment into efficacy trials of new TB vaccines and therapeutic drugs.
Copyright ©ERS 2016.
Conflict of interest statement
Conflict of interest: Disclosures can be found alongside the online version of this article at
Copyright ©ERS 2016. This version is distributed under the terms of the Creative Commons Attribution Non-Commercial Licence 4.0.
Figures
Comment in
-
Long-lasting tuberculous pleurisy.Eur Respir J. 2017 May 25;49(5):1602472. doi: 10.1183/13993003.02472-2016. Print 2017 May. Eur Respir J. 2017. PMID: 28546270 No abstract available.
-
Long-lasting tuberculous pleurisy.Eur Respir J. 2017 May 25;49(5):1700356. doi: 10.1183/13993003.00356-2017. Print 2017 May. Eur Respir J. 2017. PMID: 28546275 No abstract available.
References
-
- World Health Organization (WHO). Global Tuberculosis Report 2016. www.who.int/tb/publications/global_report/en/ Date last accessed: October 13, 2016. Date last updated: 2016.
-
- Selwyn PA, Hartel D, Lewis VA, et al. . A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med 1989; 320: 545–550. - PubMed
-
- Cantini F, Lubrano E, Marchesoni A, et al. . Latent tuberculosis infection detection and active tuberculosis prevention in patients receiving anti-TNF therapy: an Italian nationwide survey. Int J Rheum Dis 2016; 19: 799–805. - PubMed
-
- Cantini F, Niccoli L, Goletti D. Tuberculosis risk in patients treated with non-anti-tumor necrosis factor-α (TNF-α) targeted biologics and recently licensed TNF-α inhibitors: data from clinical trials and national registries. J Rheumatol Suppl 2014; 91: 56–64. - PubMed
-
- Cantini F, Niccoli L, Goletti D. Adalimumab, etanercept, infliximab, and the risk of tuberculosis: data from clinical trials, national registries, and postmarketing surveillance. J Rheumatol Suppl 2014; 91: 47–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
