Mice lacking lipid droplet-associated hydrolase, a gene linked to human prostate cancer, have normal cholesterol ester metabolism
- PMID: 27836991
- PMCID: PMC5234725
- DOI: 10.1194/jlr.M072538
Mice lacking lipid droplet-associated hydrolase, a gene linked to human prostate cancer, have normal cholesterol ester metabolism
Abstract
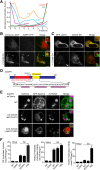
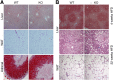
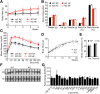
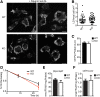
Variations in the gene LDAH (C2ORF43), which encodes lipid droplet-associated hydrolase (LDAH), are among few loci associated with human prostate cancer. Homologs of LDAH have been identified as proteins of lipid droplets (LDs). LDs are cellular organelles that store neutral lipids, such as triacylglycerols and sterol esters, as precursors for membrane components and as reservoirs of metabolic energy. LDAH is reported to hydrolyze cholesterol esters and to be important in macrophage cholesterol ester metabolism. Here, we confirm that LDAH is localized to LDs in several model systems. We generated a murine model in which Ldah is disrupted but found no evidence for a major function of LDAH in cholesterol ester or triacylglycerol metabolism in vivo, nor a role in energy or glucose metabolism. Our data suggest that LDAH is not a major cholesterol ester hydrolase, and an alternative metabolic function may be responsible for its possible effect on development of prostate cancer.
Keywords: animal models; cholesterol efflux; lipase; lipoprotein metabolism; triglycerides.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Straub B. K., Herpel E., Singer S., Zimbelmann R., Breuhahn K., Macher-Goeppinger S., Warth A., Lehmann-Koch J., Longerich T., Heid H., et al. 2010. Lipid droplet-associated PAT-proteins show frequent and differential expression in neoplastic steatogenesis. Mod. Pathol. 23: 480–492. - PubMed
-
- Hager M. H., Solomon K. R., and Freeman M. R.. 2006. The role of cholesterol in prostate cancer. Curr. Opin. Clin. Nutr. Metab. Care. 9: 379–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

