Lipid Tales of Viral Replication and Transmission
- PMID: 27838086
- PMCID: PMC5318230
- DOI: 10.1016/j.tcb.2016.09.011
Lipid Tales of Viral Replication and Transmission
Abstract
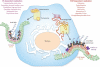
Positive-strand RNA viruses are the largest group of RNA viruses on Earth and cellular membranes are critical for all aspects of their life cycle, from entry and replication to exit. In particular, membranes serve as platforms for replication and as carriers to transmit these viruses to other cells, the latter either as an envelope surrounding a single virus or as the vesicle containing a population of viruses. Notably, many animal and human viruses appear to induce and exploit phosphatidylinositol 4-phosphate/cholesterol-enriched membranes for replication, whereas many plant and insect-vectored animal viruses utilize phosphatidylethanolamine/cholesterol-enriched membranes for the same purpose; and phosphatidylserine-enriched membrane carriers are widely used by both single and populations of viruses for transmission. Here I discuss the implications for viral pathogenesis and therapeutic development of this remarkable convergence on specific membrane lipid blueprints for replication and transmission.
Keywords: RNA virus; autophagy; cholesterol; exosome; membrane; multivesicular body; phosphatidylethanolamine; phosphatidylinositol 4-phosphate; phosphatidylserine; population.
Published by Elsevier Ltd.
Figures

Similar articles
-
Reconstitution of an RNA Virus Replicase in Artificial Giant Unilamellar Vesicles Supports Full Replication and Provides Protection for the Double-Stranded RNA Replication Intermediate.J Virol. 2020 Aug 31;94(18):e00267-20. doi: 10.1128/JVI.00267-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641477 Free PMC article.
-
The retromer is co-opted to deliver lipid enzymes for the biogenesis of lipid-enriched tombusviral replication organelles.Proc Natl Acad Sci U S A. 2021 Jan 5;118(1):e2016066118. doi: 10.1073/pnas.2016066118. Proc Natl Acad Sci U S A. 2021. PMID: 33376201 Free PMC article.
-
Extracellular vesicles: Vehicles of en bloc viral transmission.Virus Res. 2019 May;265:143-149. doi: 10.1016/j.virusres.2019.03.023. Epub 2019 Mar 27. Virus Res. 2019. PMID: 30928427 Review.
-
Modulation of the Host Lipid Landscape to Promote RNA Virus Replication: The Picornavirus Encephalomyocarditis Virus Converges on the Pathway Used by Hepatitis C Virus.PLoS Pathog. 2015 Sep 25;11(9):e1005185. doi: 10.1371/journal.ppat.1005185. eCollection 2015 Sep. PLoS Pathog. 2015. PMID: 26406250 Free PMC article.
-
Extracellular vesicles are the Trojan horses of viral infection.Curr Opin Microbiol. 2016 Aug;32:77-81. doi: 10.1016/j.mib.2016.05.004. Epub 2016 May 24. Curr Opin Microbiol. 2016. PMID: 27232382 Free PMC article. Review.
Cited by
-
Lipid Metabolism Modulation during SARS-CoV-2 Infection: A Spotlight on Extracellular Vesicles and Therapeutic Prospects.Int J Mol Sci. 2024 Jan 4;25(1):640. doi: 10.3390/ijms25010640. Int J Mol Sci. 2024. PMID: 38203811 Free PMC article. Review.
-
The enterovirus genome can be translated in an IRES-independent manner that requires the initiation factors eIF2A/eIF2D.PLoS Biol. 2023 Jan 23;21(1):e3001693. doi: 10.1371/journal.pbio.3001693. eCollection 2023 Jan. PLoS Biol. 2023. PMID: 36689548 Free PMC article.
-
Targeting host lipid flows: Exploring new antiviral and antibiotic strategies.Cell Microbiol. 2019 Mar;21(3):e12996. doi: 10.1111/cmi.12996. Epub 2019 Jan 17. Cell Microbiol. 2019. PMID: 30585688 Free PMC article. Review.
-
Perception of double-stranded RNA in plant antiviral immunity.Mol Plant Pathol. 2019 Sep;20(9):1203-1210. doi: 10.1111/mpp.12798. Epub 2019 Apr 3. Mol Plant Pathol. 2019. PMID: 30942534 Free PMC article. Review.
-
Organelles are miscommunicating: Membrane contact sites getting hijacked by pathogens.Virulence. 2023 Dec;14(1):2265095. doi: 10.1080/21505594.2023.2265095. Epub 2023 Oct 20. Virulence. 2023. PMID: 37862470 Free PMC article. Review.
References
-
- den Boon JA, Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol. 2010;64:241–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

