Dysregulation of PINCH signaling in mesial temporal epilepsy
- PMID: 27838154
- PMCID: PMC6492941
- DOI: 10.1016/j.jocn.2016.10.012
Dysregulation of PINCH signaling in mesial temporal epilepsy
Abstract
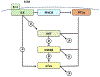
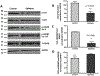
Mounting evidence suggests that inflammation is important in epileptogenesis. Particularly Interesting New Cysteine Histidine-rich (PINCH) protein is a highly conserved, LIM-domain protein known to interact with hyperphosphorylated Tau. We assessed PINCH expression in resected epileptogenic human hippocampi and further explored the relationships among PINCH, hpTau and associated kinases. Resected hippocampal tissue from 7 patients with mesial temporal lobe epilepsy (MTLE) was assessed by Western analyses to measure levels of PINCH and hyperphosphorylated Tau, as well as changes in phosphorylation levels of associated kinases AKT and GSK3β in comparison to normal control tissue. Immunolabeling was also conducted to evaluate PINCH and hpTau patterns of expression, co-localization and cell-type specific expression. Hippocampal PINCH was increased by 2.6 fold in the epilepsy cases over controls and hpTau was increased 10 fold over control. Decreased phospho-AKT and phospho-GSK3β in epilepsy tissue suggested involvement of this pathway in MTLE. PINCH and hpTau co-localized in some neurons in MTLE tissue. While PINCH was expressed by both neurons and astrocytes in MTLE tissue, hpTau was extracellular or associated with neurons. PINCH was absent from the serum of control subjects but readily detectable from the serum of patients with chronic epilepsy. Our study describes the expression of PINCH and points to AKT/GSK3β signaling dysregulation as a possible pathway in hpTau formation in MTLE. In view of the interactions between hpTau and PINCH, understanding the role of PINCH in MTLE may provide increased understanding of mechanisms leading to inflammation and MTLE epileptogenesis and a potential biomarker for drug-resistant epilepsy.
Keywords: Hippocampal sclerosis; Hyperphosphorylated Tau; Mesial temporal lobe epilepsy; PINCH protein.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures




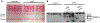
References
-
- Anthony IC, Bell JE. The Neuropathology of HIV/AIDS. Int Rev Psychiatry 2008;20:15–24. - PubMed
-
- Anthony IC, Ramage SN, Carnie FW, et al. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 2006;111:529–38. - PubMed
-
- Augustinack JC, Schneider A, Mandelkow EM, et al. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol 2002;103:26–35. - PubMed
-
- Breteler MM, de Groot RR, van Romunde LK, et al. Risk of dementia in patients with Parkinson’s disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol 1995;142:1300–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

