Neurexin, Neuroligin and Wishful Thinking coordinate synaptic cytoarchitecture and growth at neuromuscular junctions
- PMID: 27838296
- PMCID: PMC5219945
- DOI: 10.1016/j.mcn.2016.11.004
Neurexin, Neuroligin and Wishful Thinking coordinate synaptic cytoarchitecture and growth at neuromuscular junctions
Abstract
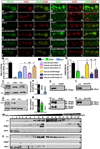
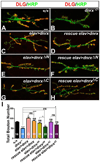
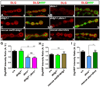
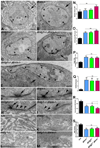
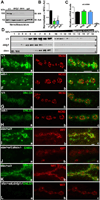
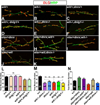
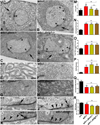
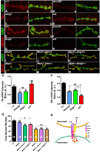
Trans-synaptic interactions involving Neurexins and Neuroligins are thought to promote adhesive interactions for precise alignment of the pre- and postsynaptic compartments and organize synaptic macromolecular complexes across species. In Drosophila, while Neurexin (Dnrx) and Neuroligins (Dnlg) are emerging as central organizing molecules at synapses, very little is known of the spectrum of proteins that might be recruited to the Dnrx/Dnlg trans-synaptic interface for organization and growth of the synapses. Using full length and truncated forms of Dnrx and Dnlg1 together with cell biological analyses and genetic interactions, we report novel functions of Dnrx and Dnlg1 in clustering of pre- and postsynaptic proteins, coordination of synaptic growth and ultrastructural organization. We show that Dnrx and Dnlg1 extracellular and intracellular regions are required for proper synaptic growth and localization of Dnlg1 and Dnrx, respectively. dnrx and dnlg1 single and double mutants display altered subcellular distribution of Discs large (Dlg), which is the homolog of mammalian post-synaptic density protein, PSD95. dnrx and dnlg1 mutants also display ultrastructural defects ranging from abnormal active zones, misformed pre- and post-synaptic areas with underdeveloped subsynaptic reticulum. Interestingly, dnrx and dnlg1 mutants have reduced levels of the Bone Morphogenetic Protein (BMP) receptor Wishful thinking (Wit), and Dnrx and Dnlg1 are required for proper localization and stability of Wit. In addition, the synaptic overgrowth phenotype resulting from the overexpression of Dnrx fails to manifest in wit mutants. Phenotypic analyses of dnrx/wit and dnlg1/wit mutants indicate that Dnrx/Dnlg1/Wit coordinate synaptic growth and architecture at the NMJ. Our findings also demonstrate that loss of Dnrx and Dnlg1 leads to decreased levels of the BMP co-receptor, Thickveins and the downstream effector phosphorylated Mad at the Neuromuscular Junction (NMJ) synapses indicating that Dnrx/Dnlg1 regulate components of the BMP signaling pathway. Together our findings reveal that Dnrx/Dnlg are at the core of a highly orchestrated process that combines adhesive and signaling mechanisms to ensure proper synaptic organization and growth during NMJ development.
Keywords: Neurexin; Neuroligin 1; Neuromuscular junctions; Subsynaptic reticulum; Synaptic growth; Wishful Thinking.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. - PubMed
-
- Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 2010;66:536–549. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

