Sustained Effectiveness of the Maternal Pertussis Immunization Program in England 3 Years Following Introduction
- PMID: 27838678
- PMCID: PMC5106626
- DOI: 10.1093/cid/ciw559
Sustained Effectiveness of the Maternal Pertussis Immunization Program in England 3 Years Following Introduction
Abstract
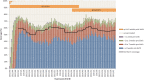
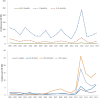
The effectiveness of maternal immunization in preventing infant pertussis was first demonstrated in England, 1 year after the program using diphtheria-tetanus-5-component acellular pertussis-inactivated polio vaccine (dT5aP-IPV) was introduced in 2012. Vaccine effectiveness against laboratory-confirmed pertussis has been sustained >90% in the 3 years following its introduction, despite changing to another acellular vaccine with different antigen composition. Consistent with this, disease incidence in infants <3 months of age has remained low despite high activity persisting in those aged 1 year and older. Vaccine effectiveness against infant deaths was estimated at 95% (95% confidence interval, 79%-100%). Additional protection from maternal immunization is retained in infants who received their first dose of the primary series. There is no longer evidence of additional protection from maternal vaccination after the third infant dose. Although numbers are small and ongoing assessment is required, there is no evidence of increased risk of disease after primary immunization in infants whose mothers received maternal vaccination.
Keywords: maternal pertussis vaccination; vaccine effectiveness.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. Revised guidance on the choice of pertussis vaccines: July 2014. Wkly Epidemiol Rec 2014; 89:337–40. - PubMed
-
- Joint Committee on Vaccination and Immunisation. Joint Committee on Vaccination and Immunisation: minutes of meeting held October 2012. Available at: https://app.box.com/s/iddfb4ppwkmtjusir2tc#/s/iddfb4ppwkmtjusir2tc/1/219.... Accessed 27 June 2016.
-
- Australian Government Department of Health. The Australian Immunisation Handbook. 10th ed, 2015. Available at: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Conte.... Accessed 27 June 2016.
-
- Vizzotti C, Neyro S, Katz N et al. . Maternal immunization in Argentina: a storyline from the prospective of a middle income country. Vaccine 2015; 33:6413–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

