Sex differences in early dyspnea relief between men and women hospitalized for acute heart failure: insights from the RELAX-AHF study
- PMID: 27838739
- PMCID: PMC5360825
- DOI: 10.1007/s00392-016-1051-4
Sex differences in early dyspnea relief between men and women hospitalized for acute heart failure: insights from the RELAX-AHF study
Abstract
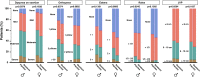
Aims: Women with heart failure are typically older, and more often have hypertension and a preserved left ventricular ejection fraction as compared with men. We sought to analyze if these sex differences influence the course and outcome of acute heart failure.
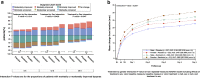
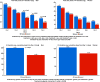
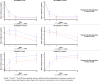
Methods and results: We analyzed sex differences in acute heart failure in 1161 patients enrolled in the RELAX-AHF study. The pre-specified study endpoints were used. At baseline, women (436/1161 patients) were older, had a higher left ventricular ejection fraction, a higher rate of hypertension, and were treated differently from men. Early dyspnea improvement (moderate or marked dyspnea improvement measured by Likert scale during the first 24 h) was greater in women. However, dyspnea improvement over the first 5 days (change from baseline in the visual analog scale area under the curve (VAS AUC) to day 5) was similar between men and women. Women reported greater improvements in general wellbeing by Likert, but no such benefits were evident with the VAS score. Multi-variable predictors of moderate or marked dyspnea improvement were female sex (p = 0.0011), lower age (p = 0.0026) and lower diuretic dose (p = 0.0067). The additional efficacy endpoints of RELAX-AHF were similar between men and women and serelaxin was equally effective in men and women.
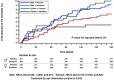
Conclusions: Women exhibit better earlier dyspnea relief and improvement in general wellbeing compared with men, even adjusted for age and left ventricular ejection fraction. However, in-hospital and post-discharge clinical outcomes were similar between men and women. This trial is registered at ClinicalTrials.gov, NCT00520806.
Keywords: Acute heart failure; Dyspnea; Gender; Serelaxin; Sex.
Conflict of interest statement
SM received consulting fees, travel support and honoraria from Servier and Novartis. JRT has received research grants or consulting fees from Amgen, Bayer, Cardio3 Bioscience, Cytokinetics, Mast Therapeutics, Medtronic, Novartis, St. Jude and Trevena. MM has received consulting income from Abbott Vascular, Bayer, Corthera, and Novartis, as well as travel support and honoraria from Servier and Novartis. PP was a consultant for Astellas, Bayer, EKR Therapeutics, J&J, the Medicines Company, Medtronic, Novartis, Otsuka, Palatin Technologies, PDL BioPharma, Pericor Therapeutics, SigmaTau, Solvay Pharmaceuticals, and Trevena; has received honoraria from Alere, Beckman-Coulter, BiogenIdec, Corthera, Ikaria, Nile Therapeutics, Momentum Research, and Overcome; has received research support from Abbott, Merck and PDL BioPharma; and has received travel support from MyLife and equipment support from Sonosite. PSP, in the last one year is or has been a Consultant for: Intersection Medical, INSYS, Janssen, Medtronic, Novartis, Trevena, scPharmaceuticals, Cardioxyl, Roche Diagnostics, Relypsa, Honoraria: Palatin Technologies. GC and BAD are employees of Momentum Research, which has provided consulting and trial management services to NovaCardia, Merck, Corthera, Novartis, Nile Therapeutics, Bioheart, Cardio3 Biosciences, Amgen, Celadon, Targegen, Trevena, Sorbent Therapeutics, and NIH. GMF reports consulting income from Novartis, Amgen, Otsuka, Trevena, Roche Diagnostics, Merck, Medtronic, Bristol Myers Squibb and grant fundings from Novartis, Amgen, Otsuka, Roche Diagnostics, and NHLBI. GF is an executive committee member and consultant to Corthera (a Novartis company), Bayer, Cardiorentis, and has received research grants from Amgen, European Union. BHG served as a consultant for Novartis, Teva, Celladon, Janssen, Zensun, Relypsa, MAST and ZS Pharma. MQ receives partial salary support from Novartis for statistical analysis of the data for this trial. TS and TAH are employees and receive salary, benefits, and stocks options from Novartis Pharma. AAV reports consultancy fees and/or research grants from: Alere, Amgen, Bayer, Boehringer Ingelheim, Cardio3Biosciences, Celladon, GSK, Merck/MSD, Novartis, Servier, Stealth Peptides, Singulex, Sphingotec, Trevena, Vifor, ZS Pharma.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

