A proof-of-concept trial of protein kinase C iota inhibition with auranofin for the paclitaxel-induced acute pain syndrome
- PMID: 27838777
- PMCID: PMC5269609
- DOI: 10.1007/s00520-016-3467-9
A proof-of-concept trial of protein kinase C iota inhibition with auranofin for the paclitaxel-induced acute pain syndrome
Abstract
Purpose: Paclitaxel causes the paclitaxel-induced acute pain (PIAP) syndrome. Based on preclinical data, we hypothesized that the protein kinase C (PKC) iota inhibitor, auranofin (a gold salt used for other pain conditions), palliates this pain.
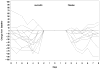
Methods: In a randomized, double-blinded manner, patients who had suffered this syndrome were assigned a one-time dose of auranofin 6 mg orally on day #2 of the chemotherapy cycle (post-paclitaxel) versus placebo. Patients completed the Brief Pain Inventory and a pain diary on days 2 through 8 and at the end of the cycle. The primary endpoint was pain scores, as calculated by area under the curve, in response to "Please rate your pain by circling the one number that best describes your pain at its worse in the last 24 hours."
Results: Thirty patients were enrolled. For the primary endpoint, mean area under the curve of 55 units (standard deviation 19) and 61 units (standard deviation 22) were observed in auranofin-treated and placebo-exposed patients, respectively (p = 0.44). On day 8 and at the end of the cycle, pain scores in auranofin-treated patients were more favorable, although differences were not statistically significant.
Conclusions: In the dose schedule studied, auranofin did not palliate the PIAP syndrome, but delayed beneficial trends suggest further study for this indication.
Keywords: Auranofin; Myalgias; Protein kinase C.
Figures

References
-
- Van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. - PubMed
-
- Eberhardt WE, Pottgen C, Gauler TC, et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy. J Clin Oncol. 2015;33:4194–201. - PubMed
-
- Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol. 2015;33:2129–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

