Pharmacokinetics, toxicity, and cytochrome P450 modulatory activity of plumbagin
- PMID: 27839515
- PMCID: PMC5108082
- DOI: 10.1186/s40360-016-0094-5
Pharmacokinetics, toxicity, and cytochrome P450 modulatory activity of plumbagin
Abstract
Background: The antimalarial activity of plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), a naturally occurring naphthoquinone widely distributed in the Plumbaginaceae family has previously been demonstrated in vitro (good activity) and in vivo (weak activity). The aim of the study was to investigate the pharmacokinetic profile following a single oral dosing to explain inconsistency of results of the in vitro and in vivo antimalarial activities. In addition, toxicity profiles and potential of modulation of cytochrome P450 enzymes (CYP1A2 and CYP3A11) were also investigated.
Methods: The pharmacokinetics and toxicity of plumbagin were investigated in rats. The propensity of plumbagin to modulate the mRNA expression and activities of the two inducible forms of hepatic drug metabolizing enzyme cytochrome P450 (CYP450), i.e., CYP1A2 and CYP3A11, was investigated using microsomes prepared from mouse livers.
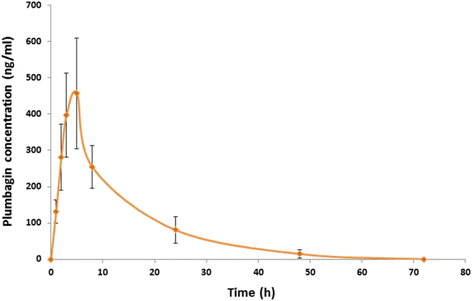
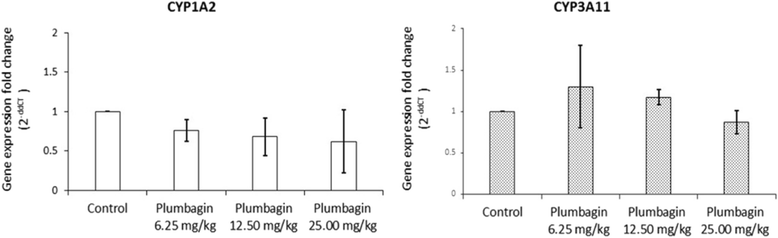
Results: Acute and subacute toxicity tests indicate low toxicity of plumbagin with maximum tolerated doses of 150 (single oral dose) and 25 (daily doses for 28 days) mg/kg body weight, respectively. The pharmacokinetic profile of plumbagin following a single oral dose of 100 mg/kg body weight suggests that delayed absorption and short residence time (median values of time to maximal concentration and elimination half-life = 9.63 and 5.0 h, respectively) in plasma. Plumbagin did not modulate mRNA expression and activities of CYP1A2 and CYP3A11.
Conclusions: Plumbagin was well tolerated following oral dose administration in rats. Pharmacokinetic property of this compound may be a limiting factor that explains the weak antimalarial activity of plumbagin observed in animal models. Potential metabolic interaction with co-administered drugs that are metabolized by CYP1A2 or CYP3A11 are unlikely.
Keywords: Antimalarial drug; Cytochrome P450; Metabolic drug interaction; Pharmacokinetics; Plumbagin; Toxicity.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

