Crystal Structure and Conformational Change Mechanism of a Bacterial Nramp-Family Divalent Metal Transporter
- PMID: 27839948
- PMCID: PMC5143219
- DOI: 10.1016/j.str.2016.09.017
Crystal Structure and Conformational Change Mechanism of a Bacterial Nramp-Family Divalent Metal Transporter
Abstract
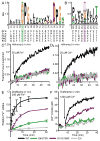
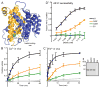
The widely conserved natural resistance-associated macrophage protein (Nramp) family of divalent metal transporters enables manganese import in bacteria and dietary iron uptake in mammals. We determined the crystal structure of the Deinococcus radiodurans Nramp homolog (DraNramp) in an inward-facing apo state, including the complete transmembrane (TM) segment 1a (absent from a previous Nramp structure). Mapping our cysteine accessibility scanning results onto this structure, we identified the metal-permeation pathway in the alternate outward-open conformation. We investigated the functional impact of two natural anemia-causing glycine-to-arginine mutations that impaired transition metal transport in both human Nramp2 and DraNramp. The TM4 G153R mutation perturbs the closing of the outward metal-permeation pathway and alters the selectivity of the conserved metal-binding site. In contrast, the TM1a G45R mutation prevents conformational change by sterically blocking the essential movement of that helix, thus locking the transporter in an inward-facing state.
Keywords: LeuT fold; MntH; Nramp; crystallography; cysteine accessibility; divalent metal transporter; microcytic anemia; natural resistance-associated macrophage protein; transition metals.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Molecular Mechanism of Nramp-Family Transition Metal Transport.J Mol Biol. 2021 Aug 6;433(16):166991. doi: 10.1016/j.jmb.2021.166991. Epub 2021 Apr 16. J Mol Biol. 2021. PMID: 33865868 Free PMC article. Review.
-
Transmembrane helix 6b links proton and metal release pathways and drives conformational change in an Nramp-family transition metal transporter.J Biol Chem. 2020 Jan 31;295(5):1212-1224. doi: 10.1074/jbc.RA119.011336. Epub 2019 Dec 27. J Biol Chem. 2020. PMID: 31882536 Free PMC article.
-
Structures in multiple conformations reveal distinct transition metal and proton pathways in an Nramp transporter.Elife. 2019 Feb 4;8:e41124. doi: 10.7554/eLife.41124. Elife. 2019. PMID: 30714568 Free PMC article.
-
Unique structural features in an Nramp metal transporter impart substrate-specific proton cotransport and a kinetic bias to favor import.J Gen Physiol. 2019 Dec 2;151(12):1413-1429. doi: 10.1085/jgp.201912428. Epub 2019 Oct 16. J Gen Physiol. 2019. PMID: 31619456 Free PMC article.
-
Recent progress in structure-function analyses of Nramp proton-dependent metal-ion transporters.Biochem Cell Biol. 2006 Dec;84(6):960-78. doi: 10.1139/o06-193. Biochem Cell Biol. 2006. PMID: 17215883 Review.
Cited by
-
Structural insights into the elevator-type transport mechanism of a bacterial ZIP metal transporter.Nat Commun. 2023 Jan 24;14(1):385. doi: 10.1038/s41467-023-36048-4. Nat Commun. 2023. PMID: 36693843 Free PMC article.
-
Structural and functional properties of a magnesium transporter of the SLC11/NRAMP family.Elife. 2022 Jan 10;11:e74589. doi: 10.7554/eLife.74589. Elife. 2022. PMID: 35001872 Free PMC article.
-
Identification of a divalent metal transporter required for cellular iron metabolism in malaria parasites.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2411631121. doi: 10.1073/pnas.2411631121. Epub 2024 Oct 28. Proc Natl Acad Sci U S A. 2024. PMID: 39467134 Free PMC article.
-
Molecular Mechanism of Nramp-Family Transition Metal Transport.J Mol Biol. 2021 Aug 6;433(16):166991. doi: 10.1016/j.jmb.2021.166991. Epub 2021 Apr 16. J Mol Biol. 2021. PMID: 33865868 Free PMC article. Review.
-
Designing yeast as plant-like hyperaccumulators for heavy metals.Nat Commun. 2019 Nov 8;10(1):5080. doi: 10.1038/s41467-019-13093-6. Nat Commun. 2019. PMID: 31704944 Free PMC article.
References
-
- Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940–959. - PubMed
-
- Barrios M, Moreno-Carralero MI, Cuadrado-Grande N, Baro M, Vivanco JL, Moran-Jimenez MJ. The homozygous mutation G75R in the human SLC11A2 gene leads to microcytic anaemia and iron overload. Br J Haematol. 2012;157:514–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

