Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy
- PMID: 27840022
- PMCID: PMC5218963
- DOI: 10.1016/j.stem.2016.09.010
Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy
Abstract
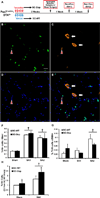
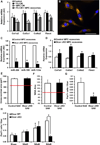
Satellite cells, the predominant stem cell population in adult skeletal muscle, are activated in response to hypertrophic stimuli and give rise to myogenic progenitor cells (MPCs) within the extracellular matrix (ECM) that surrounds myofibers. This ECM is composed largely of collagens secreted by interstitial fibrogenic cells, which influence satellite cell activity and muscle repair during hypertrophy and aging. Here we show that MPCs interact with interstitial fibrogenic cells to ensure proper ECM deposition and optimal muscle remodeling in response to hypertrophic stimuli. MPC-dependent ECM remodeling during the first week of a growth stimulus is sufficient to ensure long-term myofiber hypertrophy. MPCs secrete exosomes containing miR-206, which represses Rrbp1, a master regulator of collagen biosynthesis, in fibrogenic cells to prevent excessive ECM deposition. These findings provide insights into how skeletal stem and progenitor cells interact with other cell types to actively regulate their extracellular environments for tissue maintenance and adaptation.
Keywords: Pax7; collagen; exosomes; extracellular matrix; fibrosis; hypertrophy; microRNA; muscle; muscle progenitor cells; satellite cells.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Comment in
-
Exosomal Small Talk Carries Strong Messages from Muscle Stem Cells.Cell Stem Cell. 2017 Jan 5;20(1):1-3. doi: 10.1016/j.stem.2016.12.009. Cell Stem Cell. 2017. PMID: 28061348
References
-
- Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293:C661–C669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

