Relationship between bone turnover and density with teriparatide, denosumab or both in women in the DATA study
- PMID: 27840301
- PMCID: PMC6941193
- DOI: 10.1016/j.bone.2016.11.009
Relationship between bone turnover and density with teriparatide, denosumab or both in women in the DATA study
Abstract
Background: While changes in biochemical markers of bone turnover (BTM) have been reported to predict changes in bone mineral density (BMD), the relationship between changes in BMD and BTMs with combined antiresorptive/anabolic therapy is unknown.
Methods: In the DATA study, 94 postmenopausal osteoporotic women (ages 51-91) received either teriparatide 20-mcg SC daily, denosumab 60-mg SC every 6months, or both for 2years. Pearson's correlation coefficients (R) were calculated to determine the relationship between baseline and early changes in BTMs (as well as serum sclerostin) and 2-year changes in BMD.
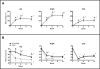
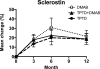
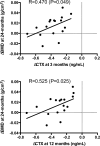
Results: In women receiving teriparatide, baseline BTMs did not correlate with 2-year BMD changes though 12-month increases in osteocalcin and P1NP were associated with 2-year increases in spine BMD. In women receiving denosumab, spine and hip BMD gains correlated with both baseline and changes in P1NP and C-telopeptide. In women receiving combined teriparatide/denosumab, while both baseline and decreases in P1NP were associated with spine BMD gains, distal radius increases were associated with less CTX suppression. Neither baseline nor changes in serum sclerostin correlated with BMD in any treatment group.
Summary and conclusions: In women treated with teriparatide or denosumab, early BTM changes (increases and decreases, respectively) predict 2-year BMD gains, especially at the spine. In women treated with combined teriparatide/denosumab therapy, BMD increases at the distal radius were associated with less suppression of bone turnover. These results suggest that efficacy of combination therapy at cortical sites such as the radius may depend on residual bone remodeling despite RANKL inhibition.
Keywords: Bone formation; Bone resorption; Bone turnover markers; Denosumab; Osteoporosis; Teriparatide.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
-
- Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20:962–70. - PubMed
-
- Blumsohn A, Marin F, Nickelsen T, Brixen K, Sigurdsson G, Gonzalez de la Vera J, Boonen S, Liu-Leage S, Barker C, Eastell R, Group ES. Early changes in biochemical markers of bone turnover and their relationship with bone mineral density changes after 24 months of treatment with teriparatide. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22:1935–46. - PMC - PubMed
-
- Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH. PINP as an aid for monitoring patients treated with teriparatide. Bone. 2011;48:798–803. - PubMed
-
- Eastell R, Christiansen C, Grauer A, Kutilek S, Libanati C, McClung MR, Reid IR, Resch H, Siris E, Uebelhart D, Wang A, Weryha G, Cummings SR. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:530–7. - PubMed
-
- Leder BZ, Tsai JN, Uihlein AV, Burnett-Bowie SA, Zhu Y, Foley K, Lee H, Neer RM. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. The Journal of clinical endocrinology and metabolism. 2014;99:1694–700. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

