Rheum emodin inhibits enterovirus 71 viral replication and affects the host cell cycle environment
- PMID: 27840410
- PMCID: PMC5342659
- DOI: 10.1038/aps.2016.110
Rheum emodin inhibits enterovirus 71 viral replication and affects the host cell cycle environment
Abstract
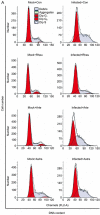
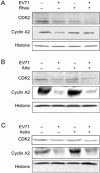
Human enterovirus 71 (EV71) is the primary causative agent of recent large-scale outbreaks of hand, foot, and mouth disease (HFMD) in Asia. Currently, there are no drugs available for the prevention and treatment of HFMD. In this study, we compared the anti-EV71 activities of three natural compounds, rheum emodin, artemisinin and astragaloside extracted from Chinese herbs Chinese rhubarb, Artemisia carvifolia and Astragalus, respectively, which have been traditionally used for the treatment and prevention of epidemic diseases. Human lung fibroblast cell line MRC5 was mock-infected or infected with EV71, and treated with drugs. The cytotoxicity of the drugs was detected with MTT assay. The cytopathic effects such as cell death and condensed nuclei were morphologically observed. The VP1-coding sequence required for EV71 genome replication was assayed with qRT-PCR. Viral protein expression was analyzed with Western blotting. Viral TCID50 was determined to evaluate EV71 virulence. Flow cytometry analysis of propidium iodide staining was performed to analyze the cell cycle distribution of MRC5 cells. Rheum emodin (29.6 μmol/L) effectively protected MRC5 cells from EV71-induced cytopathic effects, which resulted from the inhibiting viral replication: rheum emodin treatment decreased viral genomic levels by 5.34-fold, viral protein expression by less than 30-fold and EV71 virulence by 0.33107-fold. The fact that inhibition of rheum emodin on viral virulence was much stronger than its effects on genomic levels and viral protein expression suggested that rheum emodin inhibited viral maturation. Furthermore, rheum emodin treatment markedly diminished cell cycle arrest at S phase in MRC5 cells, which was induced by EV71 infection and favored the viral replication. In contrast, neither astragaloside (50 μmol/L) nor artemisinin (50 μmol/L) showed similar anti-EV71 activities. Among the three natural compounds tested, rheum emodin effectively suppressed EV71 viral replication, thus is a candidate anti-HFMD drug.
Figures




Similar articles
-
Glycyrrhizic acid as the antiviral component of Glycyrrhiza uralensis Fisch. against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease.J Ethnopharmacol. 2013 May 2;147(1):114-21. doi: 10.1016/j.jep.2013.02.017. Epub 2013 Feb 27. J Ethnopharmacol. 2013. PMID: 23454684 Free PMC article.
-
Pyrrolidine dithiocarbamate inhibits enterovirus 71 replication by down-regulating ubiquitin-proteasome system.Virus Res. 2015 Jan 2;195:207-16. doi: 10.1016/j.virusres.2014.10.012. Epub 2014 Oct 23. Virus Res. 2015. PMID: 25456405
-
Salvianolic Acid B Inhibits Hand-Foot-Mouth Disease Enterovirus 71 Replication through Enhancement of AKT Signaling Pathway.J Microbiol Biotechnol. 2020 Jan 28;30(1):38-43. doi: 10.4014/jmb.1907.07079. J Microbiol Biotechnol. 2020. PMID: 31752055 Free PMC article.
-
[Research into the Pathogenicity of Enterovirus 71].Bing Du Xue Bao. 2015 Mar;31(2):192-6. Bing Du Xue Bao. 2015. PMID: 26164947 Review. Chinese.
-
Strategies to develop antivirals against enterovirus 71.Virol J. 2013 Jan 22;10:28. doi: 10.1186/1743-422X-10-28. Virol J. 2013. PMID: 23339605 Free PMC article. Review.
Cited by
-
Advances in the study of emodin: an update on pharmacological properties and mechanistic basis.Chin Med. 2021 Oct 10;16(1):102. doi: 10.1186/s13020-021-00509-z. Chin Med. 2021. PMID: 34629100 Free PMC article. Review.
-
Characterization of Anti-Poliovirus Compounds Isolated from Edible Plants.Viruses. 2023 Mar 31;15(4):903. doi: 10.3390/v15040903. Viruses. 2023. PMID: 37112883 Free PMC article.
-
Enterovirus E infections in goats with respiratory disease.BMC Vet Res. 2025 Feb 17;21(1):71. doi: 10.1186/s12917-025-04537-x. BMC Vet Res. 2025. PMID: 39962514 Free PMC article.
-
The Inhibitory Activity of Anthraquinones against Pathogenic Protozoa, Bacteria, and Fungi and the Relationship to Structure.Molecules. 2020 Jul 7;25(13):3101. doi: 10.3390/molecules25133101. Molecules. 2020. PMID: 32646028 Free PMC article.
-
Isolation and Identification of Two Clinical Strains of the Novel Genotype Enterovirus E5 in China.Microbiol Spectr. 2022 Jun 29;10(3):e0266221. doi: 10.1128/spectrum.02662-21. Epub 2022 Jun 2. Microbiol Spectr. 2022. PMID: 35652637 Free PMC article.
References
-
- Fan X, Jiang J, Liu Y, Huang X, Wang P, Liu L, et al. Detection of human enterovirus 71 and Coxsackievirus A16 in an outbreak of hand, foot, and mouth disease in Henan Province, China in 2009. Virus Genes 2012; 46: 1–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

