Optical Coherence Tomography as a Biomarker for Diagnosis, Progression, and Prognosis of Neurodegenerative Diseases
- PMID: 27840739
- PMCID: PMC5093273
- DOI: 10.1155/2016/8503859
Optical Coherence Tomography as a Biomarker for Diagnosis, Progression, and Prognosis of Neurodegenerative Diseases
Abstract
Neurodegenerative diseases present a current challenge for accurate diagnosis and for providing precise prognostic information. Developing imaging biomarkers for multiple sclerosis (MS), Parkinson disease (PD), and Alzheimer's disease (AD) will improve the clinical management of these patients and may be useful for monitoring treatment effectiveness. Recent research using optical coherence tomography (OCT) has demonstrated that parameters provided by this technology may be used as potential biomarkers for MS, PD, and AD. Retinal thinning has been observed in these patients and new segmentation software for the analysis of the different retinal layers may provide accurate information on disease progression and prognosis. In this review we analyze the application of retinal evaluation using OCT technology to provide better understanding of the possible role of the retinal layers thickness as biomarker for the detection of these neurodegenerative pathologies. Current OCT analysis of the retinal nerve fiber layer and, specially, the ganglion cell layer thickness may be considered as a good biomarker for disease diagnosis, severity, and progression.
Figures
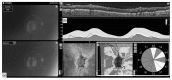
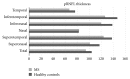
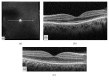
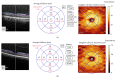
References
-
- García-Martín E., Pueyo V., Martin J., et al. Progressive changes in the retinal nerve fiber layer in patients with multiple sclerosis. European Journal of Ophthalmology. 2010;20(1):167–173. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

