Visible and Near-Infrared Photothermal Catalyzed Hydrogenation of Gaseous CO2 over Nanostructured Pd@Nb2O5
- PMID: 27840802
- PMCID: PMC5095794
- DOI: 10.1002/advs.201600189
Visible and Near-Infrared Photothermal Catalyzed Hydrogenation of Gaseous CO2 over Nanostructured Pd@Nb2O5
Abstract
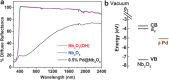
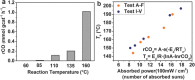
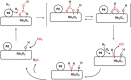
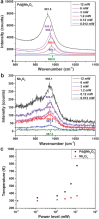
The reverse water gas shift (RWGS) reaction driven by Nb2O5 nanorod-supported Pd nanocrystals without external heating using visible and near infrared (NIR) light is demonstrated. By measuring the dependence of the RWGS reaction rates on the intensity and spectral power distribution of filtered light incident onto the nanostructured Pd@Nb2O5 catalyst, it is determined that the RWGS reaction is activated photothermally. That is the RWGS reaction is initiated by heat generated from thermalization of charge carriers in the Pd nanocrystals that are excited by interband and intraband absorption of visible and NIR light. Taking advantage of this photothermal effect, a visible and NIR responsive Pd@Nb2O5 hybrid catalyst that efficiently hydrogenates CO2 to CO at an impressive rate as high as 1.8 mmol gcat-1 h-1 is developed. The mechanism of this photothermal reaction involves H2 dissociation on Pd nanocrystals and subsequent spillover of H to the Nb2O5 nanorods whereupon adsorbed CO2 is hydrogenated to CO. This work represents a significant enhancement in our understanding of the underlying mechanism of photothermally driven CO2 reduction and will help guide the way toward the development of highly efficient catalysts that exploit the full solar spectrum to convert gas-phase CO2 to valuable chemicals and fuels.
Keywords: carbon dioxide; niobium oxide nanostructure; palladium catalysts; photothermal catalysis; solar fuels.
Figures




References
-
- Herron J. A., Kim J., Upadhye A. A., Huber G. W., Maravelias C. T., Energy Environ. Sci. 2015, 8, 126.
-
- a) Zhou H., Fan T., Zhang D., ChemCatChem 2011, 3, 513;
- b) Tran P. D., Wong L. H., Barber J., Loo J. S. C., Energy Environ. Sci. 2012, 5, 5902;
- c) Liu L., Gao F., Zhao H., Li Y., Appl. Catal., B 2013, 134, 349;
- d) Kumar B., Llorente M., Froehlich J., Dang T., Sathrum A., Kubiak C. P., Annu. Rev. Phys. Chem. 2012, 63, 541; - PubMed
- e) Dasgupta S., Brunschwig B. S., Winkler J. R., Gray H. B., Chem. Soc. Rev. 2013, 42, 2213; - PubMed
- f) Zhao X., Luo B., Long R., Wang C., Xiong Y., J. Mater. Chem. A 2015, 3, 4134.
-
- Tu W., Zhou Y., Zou Z., Adv. Mater. 2014, 26, 4607. - PubMed
-
- a) Yan S., Wang J., Gao H., Wang N., Yu H., Li Z., Zhou Y., Zou Z., Adv. Funct. Mater. 2013, 23, 1839;
- b) Teramura K., Iguchi S., Mizuno Y., Shishido T., Tanaka T., Angew. Chem. Int. Ed. 2012, 124, 8132; - PubMed
- c) Ahmed N., Morikawa M., Izumi Y., Catal. Today 2012, 185, 263;
- d) Teramura K., Tanaka T., Ishikawa H., Kohno Y., Funabiki T., J. Phys. Chem. B 2004, 108, 346;
- e) Zhao Y., Chen G., Bian T., Zhou C., Waterhouse G. I. N., Wu L.‐Z., Tung C.‐H., Smith L. J., O'Hare D., Zhang T., Adv. Mater. 2015, 27, 7824; - PubMed
- f) Li R., Hu J., Deng M., Wang H., Wang X., Hu Y., Jiang H.‐L., Jiang J., Zhang Q., Xie Y., Xiong Y., Adv. Mater. 2014, 26, 4783; - PubMed
- g) Zhao Y., Jia X., Waterhouse G. I. N., Wu L.‐Z., Tung C.‐H., O'Hare D., Zhang T., Adv. Energy Mater. 2016, 6, 1501974.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
