Identification of HLA‑A*1101‑restricted cytotoxic T lymphocyte epitopes derived from epidermal growth factor pathway substrate number 8
- PMID: 27840923
- PMCID: PMC5355652
- DOI: 10.3892/mmr.2016.5888
Identification of HLA‑A*1101‑restricted cytotoxic T lymphocyte epitopes derived from epidermal growth factor pathway substrate number 8
Abstract
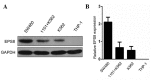
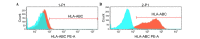
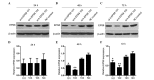
Epidermal growth factor receptor pathway substrate 8 (EPS8) is critical in the proliferation, progression and metastasis of solid and hematological types of cancer, and thus constitutes an ideal target for cancer immunotherapy. The present study aimed to identify human leukocyte antigen (HLA)‑A*1101‑restricted cytotoxic T lymphocyte (CTL) epitopes from EPS8 and characterize their immunotherapeutic efficacy in vitro. Two computer‑based algorithms were used to predict native EPS8 epitopes with potential high binding affinity to the HLA‑A*1101 molecule, which is the HLA‑A allele with the highest frequency in the Chinese population. The peptide‑induced cytokine production from the CTLs was examined using enzyme‑linked immunosorbent spot analysis. The cytotoxic effects on cancer cells by CTLs primed with the identified peptides were examined using flow cytometry. A total of five peptides, designated as P380, P70, P82, P30 and P529, presented with high affinity towards the HLA‑A*1101 molecule. In response to stimulation by these five peptides, enhanced secretion of interferon‑γ from the CTLs and increased cytolytic capabilities of the CTLs toward cancer cells were noted, with the most potent effects observed from the P380 peptide. Taken together, the present study identified five potential CTL epitopes from EPS8. Among these, P380 presented with the highest therapeutic efficacy in vitro. These peptides may benefit the development of EPS8‑based immunotherapy for the treatment of HLA‑A*1101‑positive hematological malignancies.
Figures


References
-
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. - DOI - PubMed
-
- Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, Durrant IJ, Luger SM, Marks DI, Franklin IM, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. - DOI - PubMed
-
- Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, Stadler M, Kuball J, Cornelissen J, Vorlicek J, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–1606. doi: 10.1182/blood-2011-08-375840. - DOI - PubMed
-
- Mohamed YS, Dunnion D, Teobald I, Walewska R, Browning MJ. In vitro evaluation of human hybrid cell lines generated by fusion of B-lymphoblastoid cells and ex vivo tumour cells as candidate vaccines for haematological malignancies. Vaccine. 2012;30:6578–6587. doi: 10.1016/j.vaccine.2012.08.032. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

