Tangshen formula attenuates hepatic steatosis by inhibiting hepatic lipogenesis and augmenting fatty acid oxidation in db/db mice
- PMID: 27840945
- PMCID: PMC5117754
- DOI: 10.3892/ijmm.2016.2799
Tangshen formula attenuates hepatic steatosis by inhibiting hepatic lipogenesis and augmenting fatty acid oxidation in db/db mice
Abstract
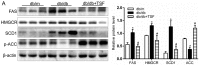
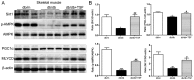
Tangshen formula (TSF), a well-prescribed traditional Chinese formula, has been used in the treatment of diabetic nephropathy. However, whether TSF ameliorates dyslipidemia and liver injury associated with diabetes remains unclear. In this study, we examined the effects of TSF on lipid profiles and hepatic steatosis in db/db mice. For this purpose, 8‑week-old db/db mice were treated with TSF or saline for 12 weeks via gavage and db/m mice were used as controls. Body weight and blood glucose levels were monitored weekly and bi-weekly, respectively. Blood samples were obtained for the analysis of lipids and enzymes related to hepatic function, and liver tissues were analyzed by histology, immunohistochemistry and molecular examination. The results revealed that TSF markedly reduced body weight, liver index [liver/body weight (LW/BW)] and improved lipid profiles, hepatic function and steatosis in db/db mice. TSF induced the phosphoralation of AMP-activated protein kinase and inhibited the activity of sterol regulatory element-binding protein 1 together with the inhibition of the expression of genes involved in de novo lipogenesis (DNL) and gluconeogenesis, such as fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC), stearoyl CoA desaturase 1 (SCD1), glucose-6-phosphatase (G6pc) and phosphoenolpyruvate carboxykinase 1 (Pck1). Additionally, the silent mating type information regulation 2 homolog 1 (Sirt1)/peroxisome proliferator-activated receptor α (PPARα)/malonyl-CoA decarboxylase (MLYCD) cascade was potently activated by TSF in the liver and skeletal muscle of db/db mice, which led to enhanced fatty acid oxidation. These findings demonstrated that TSF attenuated hepatic fat accumulation and steatosis in db/db mice by inhibiting lipogenesis and augmenting fatty acid oxidation.
Figures











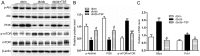
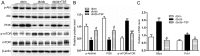

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

