Aflibercept and Ang1 supplementation improve neoadjuvant or adjuvant chemotherapy in a preclinical model of resectable breast cancer
- PMID: 27841282
- PMCID: PMC5107907
- DOI: 10.1038/srep36694
Aflibercept and Ang1 supplementation improve neoadjuvant or adjuvant chemotherapy in a preclinical model of resectable breast cancer
Abstract
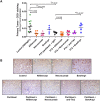
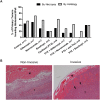
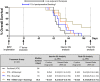
Phase III clinical trials evaluating bevacizumab (an antibody to the angiogenic ligand, VEGF-A) in breast cancer have found improved responses in the presurgical neoadjuvant setting but no benefits in the postsurgical adjuvant setting. The objective of this study was to evaluate alternative antiangiogenic therapies, which target multiple VEGF family members or differentially modulate the Angiopoietin/Tie2 pathway, in a mouse model of resectable triple-negative breast cancer (TNBC). Neoadjuvant therapy experiments involved treating established orthotopic xenografts of an aggressive metastatic variant of the MDA-MB-231 human TNBC cell line, LM2-4. Adjuvant therapies were given after primary tumor resections to treat postsurgical regrowths and distant metastases. Aflibercept ('VEGF Trap', which neutralizes VEGF-A, VEGF-B and PlGF) showed greater efficacy than nesvacumab (an anti-Ang2 antibody) as an add-on to neoadjuvant/adjuvant chemotherapy. Concurrent inhibition of Ang1 and Ang2 signaling (through an antagonistic anti-Tie2 antibody) was not more efficacious than selective Ang2 inhibition. In contrast, short-term perioperative BowAng1 (a recombinant Ang1 variant) improved the efficacy of adjuvant chemotherapy. In conclusion, concurrent VEGF pathway inhibition is more likely than Ang/Tie2 pathway inhibition (e.g., anti-Ang2, anti-Ang2/Ang1, anti-Tie2) to improve neoadjuvant/adjuvant chemotherapies for TNBC. Short-term perioperative Ang1 supplementation may also have therapeutic potential in conjunction with adjuvant chemotherapy for TNBC.
Conflict of interest statement
G.T. is an employee of Regeneron. R.S.K. and F.T.H.W. received honoraria recently from Regeneron. R.S.K. is a member of Scientific Advisory Board of Angiocrine Bioscience Inc. and MolMed Inc., a consultant to Triphase Accelerator LLC, and has received honoraria recently from Boehringer-Ingelheim, Eli Lilly and Neovacs Pharma.
Figures


Similar articles
-
Ang2 inhibitors and Tie2 activators: potential therapeutics in perioperative treatment of early stage cancer.EMBO Mol Med. 2021 Jul 7;13(7):e08253. doi: 10.15252/emmm.201708253. Epub 2021 Jun 14. EMBO Mol Med. 2021. PMID: 34125494 Free PMC article. Review.
-
Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody.Mol Cancer Ther. 2010 Oct;9(10):2641-51. doi: 10.1158/1535-7163.MCT-10-0213. Mol Cancer Ther. 2010. PMID: 20937592 Free PMC article.
-
Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization.Circ Res. 1998 Aug 10;83(3):233-40. doi: 10.1161/01.res.83.3.233. Circ Res. 1998. PMID: 9710115
-
Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition.Cancer Res. 2013 Jan 1;73(1):108-18. doi: 10.1158/0008-5472.CAN-12-2064. Epub 2012 Nov 13. Cancer Res. 2013. PMID: 23149917
-
Preclinical recapitulation of antiangiogenic drug clinical efficacies using models of early or late stage breast cancer metastatis.Breast. 2013 Aug;22 Suppl 2:S57-65. doi: 10.1016/j.breast.2013.07.011. Breast. 2013. PMID: 24074794 Review.
Cited by
-
Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies.Trends Mol Med. 2021 Apr;27(4):314-331. doi: 10.1016/j.molmed.2020.11.006. Epub 2020 Dec 10. Trends Mol Med. 2021. PMID: 33309601 Free PMC article. Review.
-
Durability of cell line xenograft resection models to interrogate tumor micro-environment targeting agents.Sci Rep. 2019 Jun 24;9(1):9204. doi: 10.1038/s41598-019-45444-0. Sci Rep. 2019. PMID: 31235775 Free PMC article.
-
Inhibition of NF-κB signaling pathway induces apoptosis and suppresses proliferation and angiogenesis of human fibroblast-like synovial cells in rheumatoid arthritis.Medicine (Baltimore). 2018 Jun;97(23):e10920. doi: 10.1097/MD.0000000000010920. Medicine (Baltimore). 2018. PMID: 29879032 Free PMC article.
-
Ang2 inhibitors and Tie2 activators: potential therapeutics in perioperative treatment of early stage cancer.EMBO Mol Med. 2021 Jul 7;13(7):e08253. doi: 10.15252/emmm.201708253. Epub 2021 Jun 14. EMBO Mol Med. 2021. PMID: 34125494 Free PMC article. Review.
-
HEYL Regulates Neoangiogenesis Through Overexpression in Both Breast Tumor Epithelium and Endothelium.Front Oncol. 2021 Jan 15;10:581459. doi: 10.3389/fonc.2020.581459. eCollection 2020. Front Oncol. 2021. PMID: 33520697 Free PMC article.
References
-
- Jayson G. C., Kerbel R., Ellis L. M. & Harris A. L. Antiangiogenic therapy in oncology: current status and future directions. Lancet 388, 518–529 (2016). - PubMed
-
- Montero A. J. & Vogel C. Fighting fire with fire: rekindling the bevacizumab debate. N. Engl. J. Med. 366, 374–375 (2012). - PubMed
-
- Bartsch R., Gnant M. & Steger G. G. Bevacizumab: no comeback in early breast cancer? Lancet Oncol. 16, 1001 1003 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

