TRF1 phosphorylation on T271 modulates telomerase-dependent telomere length maintenance as well as the formation of ALT-associated PML bodies
- PMID: 27841304
- PMCID: PMC5107961
- DOI: 10.1038/srep36913
TRF1 phosphorylation on T271 modulates telomerase-dependent telomere length maintenance as well as the formation of ALT-associated PML bodies
Abstract
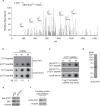
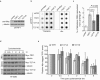
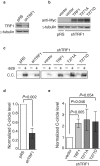
TRF1, a component of the shelterin complex, plays a key role in both telomerase-dependent telomere maintenance and alternative lengthening of telomeres, the latter also known as ALT. Characteristics of ALT cells include C-circles and ALT-associated PML bodies, referred to as APBs. The function of TRF1 is tightly regulated by post-translational modification including phosphorylation, however TRF1 phosphorylation sites have yet to be fully characterized. Here we report a novel TRF1 phosphorylation site threonine 271. We show that a nonphosphorylatable mutation of T271A impairs TRF1 binding to telomeric DNA in vivo and renders TRF1 defective in inhibiting telomerase-dependent telomere elongation. On the other hand, TRF1 carrying a phosphomimic mutation of T271D is competent in not only binding to telomeric DNA but also inhibiting telomerase-mediated telomere lengthening. These results suggest that TRF1 phosphorylation on T271 negatively regulates telomerase-mediated telomere maintenance. We find that in telomerase-negative ALT cells, TRF1 carrying either a T271A or T271D mutation is able to promote C-circle production but fails to support APB formation. These results suggest that TRF1 phosphorylation on T271 is necessary for APB formation but dispensable for C-circle production. These results further imply that APB formation can be mechanistically separated from C-circle production.
Figures


Similar articles
-
The F-box protein β-TrCP promotes ubiquitination of TRF1 and regulates the ALT-associated PML bodies formation in U2OS cells.Biochem Biophys Res Commun. 2013 May 17;434(4):728-34. doi: 10.1016/j.bbrc.2013.03.096. Epub 2013 Apr 10. Biochem Biophys Res Commun. 2013. PMID: 23583392
-
Cdk-dependent phosphorylation regulates TRF1 recruitment to PML bodies and promotes C-circle production in ALT cells.J Cell Sci. 2016 Jul 1;129(13):2559-72. doi: 10.1242/jcs.186098. Epub 2016 May 16. J Cell Sci. 2016. PMID: 27185864
-
De novo assembly of a PML nuclear subcompartment occurs through multiple pathways and induces telomere elongation.J Cell Sci. 2011 Nov 1;124(Pt 21):3603-18. doi: 10.1242/jcs.084681. Epub 2011 Nov 1. J Cell Sci. 2011. PMID: 22045732
-
Post-translational modifications of TRF1 and TRF2 and their roles in telomere maintenance.Mech Ageing Dev. 2012 Jun;133(6):421-34. doi: 10.1016/j.mad.2012.05.002. Epub 2012 May 23. Mech Ageing Dev. 2012. PMID: 22634377 Review.
-
Role of Pin2/TRF1 in telomere maintenance and cell cycle control.J Cell Biochem. 2003 May 1;89(1):19-37. doi: 10.1002/jcb.10496. J Cell Biochem. 2003. PMID: 12682905 Review.
Cited by
-
Loci for human leukocyte telomere length in the Singaporean Chinese population and trans-ethnic genetic studies.Nat Commun. 2019 Jun 6;10(1):2491. doi: 10.1038/s41467-019-10443-2. Nat Commun. 2019. PMID: 31171785 Free PMC article.
-
Diagnosis and treatment of ALT tumors: is Trabectedin a new therapeutic option?J Exp Clin Cancer Res. 2017 Dec 22;36(1):189. doi: 10.1186/s13046-017-0657-3. J Exp Clin Cancer Res. 2017. PMID: 29273061 Free PMC article. Review.
-
Modulation of telomere protection by the PI3K/AKT pathway.Nat Commun. 2017 Nov 2;8(1):1278. doi: 10.1038/s41467-017-01329-2. Nat Commun. 2017. PMID: 29097657 Free PMC article.
-
RNA 5-methylcytosine status is associated with DNMT2/TRDMT1 nuclear localization in osteosarcoma cell lines.J Bone Oncol. 2022 Jul 30;36:100448. doi: 10.1016/j.jbo.2022.100448. eCollection 2022 Oct. J Bone Oncol. 2022. PMID: 35942470 Free PMC article.
-
Epigenetic Regulation of Telomere Maintenance for Therapeutic Interventions in Gliomas.Genes (Basel). 2017 May 17;8(5):145. doi: 10.3390/genes8050145. Genes (Basel). 2017. PMID: 28513547 Free PMC article. Review.
References
-
- Levy M. Z., Allsopp R. C., Futcher A. B., Greider C. W. & Harley C. B. Telomere end-replication problem and cell aging. J. Mol. Biol. 225, 951–960 (1992). - PubMed
-
- d’Adda di Fagagna F. et al.. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003). - PubMed
-
- Shay J. W. & Bacchetti S. A survey of telomerase activity in human cancer. Eur. J. Cancer 33, 787–791 (1997). - PubMed
-
- Cesare A. J. & Reddel R. R. Alternative lengthening of telomeres: models, mechanisms and implications. Nat. Rev. Genet. 11, 319–330 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

