Glycinergic dysfunction in a subpopulation of dorsal horn interneurons in a rat model of neuropathic pain
- PMID: 27841371
- PMCID: PMC5107903
- DOI: 10.1038/srep37104
Glycinergic dysfunction in a subpopulation of dorsal horn interneurons in a rat model of neuropathic pain
Abstract
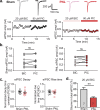
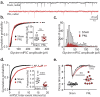
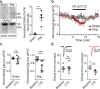
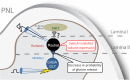
The development of neuropathic pain involves persistent changes in signalling within pain pathways. Reduced inhibitory signalling in the spinal cord following nerve-injury has been used to explain sensory signs of neuropathic pain but specific circuits that lose inhibitory input have not been identified. This study shows a specific population of spinal cord interneurons, radial neurons, lose glycinergic inhibitory input in a rat partial sciatic nerve ligation (PNL) model of neuropathic pain. Radial neurons are excitatory neurons located in lamina II of the dorsal horn, and are readily identified by their morphology. The amplitude of electrically-evoked glycinergic inhibitory post-synaptic currents (eIPSCs) was greatly reduced in radial neurons following nerve-injury associated with increased paired-pulse ratio. There was also a reduction in frequency of spontaneous IPSCs (sIPSCs) and miniature IPSCs (mIPSC) in radial neurons without significantly affecting mIPSC amplitude. A subtype selective receptor antagonist and western blots established reversion to expression of the immature glycine receptor subunit GlyRα2 in radial neurons after PNL, consistent with slowed decay times of IPSCs. This study has important implications as it identifies a glycinergic synaptic connection in a specific population of dorsal horn neurons where loss of inhibitory signalling may contribute to signs of neuropathic pain.
Figures




References
-
- Niikura K., Narita M., Butelman E. R., Kreek M. J. & Suzuki T. Neuropathic and chronic pain stimuli downregulate central mu-opioid and dopaminergic transmission. Trends Pharmacol Sci. 31, 299–305 (2010). - PubMed
-
- Gilron I., Baron R. & Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 90, 532–545 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

