Lamotrigine versus carbamazepine monotherapy for epilepsy: an individual participant data review
- PMID: 27841445
- PMCID: PMC6478073
- DOI: 10.1002/14651858.CD001031.pub3
Lamotrigine versus carbamazepine monotherapy for epilepsy: an individual participant data review
Update in
-
Lamotrigine versus carbamazepine monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2018 Jun 28;6(6):CD001031. doi: 10.1002/14651858.CD001031.pub4. Cochrane Database Syst Rev. 2018. PMID: 29952431 Free PMC article.
Abstract
Background: This is an updated version of the original Cochrane review published in Issue 1, 2006 of the Cochrane Database of Systematic Reviews.Epilepsy is a common neurological condition in which abnormal electrical discharges from the brain cause recurrent unprovoked seizures. It is believed that with effective drug treatment up to 70% of individuals with active epilepsy have the potential to become seizure-free and to go into long-term remission shortly after starting drug therapy with a single antiepileptic drug (AED) in monotherapy.The correct choice of first-line antiepileptic therapy for individuals with newly diagnosed seizures is of great importance. It is important that the choice of AEDs for an individual is made using the highest quality evidence regarding the potential benefits and harms of the various treatments. It is also important that the effectiveness and tolerability of AEDs appropriate to given seizure types are compared to one another.Carbamazepine or lamotrigine are first-line recommended treatments for new onset partial seizures and as a first- or second-line treatment for generalised tonic-clonic seizures. Performing a synthesis of the evidence from existing trials will increase the precision of the results for outcomes relating to efficacy and tolerability and may assist in informing a choice between the two drugs.
Objectives: To review the time to withdrawal, remission and first seizure with lamotrigine compared to carbamazepine when used as monotherapy in people with partial onset seizures (simple or complex partial and secondarily generalised) or generalised onset tonic-clonic seizures (with or without other generalised seizure types).
Search methods: The first searches for this review were run in 1997. For the most recent update we searched the Cochrane Epilepsy Group Specialized Register (17 October 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 17 October 2016) and MEDLINE (Ovid, 1946 to 17 October 2016). We imposed no language restrictions. We also contacted pharmaceutical companies and trial investigators.
Selection criteria: Randomised controlled trials in children or adults with partial onset seizures or generalised onset tonic-clonic seizures comparing monotherapy with either carbamazepine or lamotrigine.
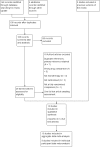
Data collection and analysis: This was an individual participant data (IPD) review. Our primary outcome was time to withdrawal of allocated treatment and our secondary outcomes were time to first seizure post-randomisation, time to six-month, 12-month and 24-month remission, and incidence of adverse events. We used Cox proportional hazards regression models to obtain trial-specific estimates of hazard ratios (HRs) with 95% confidence intervals (CIs), using the generic inverse variance method to obtain the overall pooled HR and 95% CI.
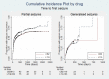
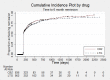
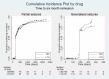
Main results: We included 13 studies in this review. Individual participant data were available for 2572 participants out of 3394 eligible individuals from nine out of 13 trials: 78% of the potential data. For remission outcomes, a HR < 1 indicated an advantage for carbamazepine and for first seizure and withdrawal outcomes a HR < 1 indicated an advantage for lamotrigine.The main overall results (pooled HR adjusted for seizure type) were: time to withdrawal of allocated treatment (HR 0.72, 95% CI 0.63 to 0.82), time to first seizure (HR 1.22, 95% CI 1.09 to 1.37) and time to six-month remission (HR 0.84, 95% CI 0.74 to 0.94), showing a significant advantage for lamotrigine compared to carbamazepine for withdrawal but a significant advantage for carbamazepine compared to lamotrigine for first seizure and six-month remission. We found no difference between the drugs for time to 12-month remission (HR 0.91, 95% CI 0.77 to 1.07) or time to 24-month remission (HR 1.00, 95% CI 0.80 to 1.25), however only two trials followed up participants for more than one year so the evidence is limited.The results of this review are applicable mainly to individuals with partial onset seizures; 88% of included individuals experienced seizures of this type at baseline. Up to 50% of the limited number of individuals classified as experiencing generalised onset seizures at baseline may have had their seizure type misclassified, therefore we recommend caution when interpreting the results of this review for individuals with generalised onset seizures.The most commonly reported adverse events for both of the drugs across all of the included trials were dizziness, fatigue, gastrointestinal disturbances, headache and skin problems. The rate of adverse events was similar across the two drugs.The methodological quality of the included trials was generally good, however there is some evidence that the design choice of masked or open-label treatment may have influenced the withdrawal rates of the trials. Hence, we judged the quality of the evidence for the primary outcome of treatment withdrawal to be moderate for individuals with partial onset seizures and low for individuals with generalised onset seizures. For efficacy outcomes (first seizure, remission), we judged the quality of evidence to be high for individuals with partial onset seizures and moderate for individuals with generalised onset seizures.
Authors' conclusions: Lamotrigine was significantly less likely to be withdrawn than carbamazepine but the results for time to first seizure suggested that carbamazepine may be superior in terms of seizure control. A choice between these first-line treatments must be made with careful consideration. We recommend that future trials should be designed to the highest quality possible with consideration of masking, choice of population, classification of seizure type, duration of follow-up, choice of outcomes and analysis, and presentation of results.
Conflict of interest statement
SJ Nolan: none known.
J Weston: none known.
AG Marson was Chief Investigator of SANAD A 2007. A consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Tony Marson is Theme Leader for Managing Complex Needs at NIHR CLAHRC NWC.
C Tudur Smith was involved in the statistical analysis of SANAD A 2007.
Figures




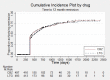
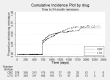










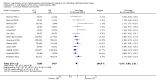




Update of
-
Lamotrigine versus carbamazepine monotherapy for epilepsy.Cochrane Database Syst Rev. 2006 Jan 25;(1):CD001031. doi: 10.1002/14651858.CD001031.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2016 Nov 14;11:CD001031. doi: 10.1002/14651858.CD001031.pub3. PMID: 16437428 Updated.
References
References to studies included in this review
-
- Brodie MJ, Richens A, Yuen AWC, for UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Double‐blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. Lancet 1995;345(8948):476‐9. - PubMed
- Gillham R. Use of SEALS, a quality of life instrument, in evaluating lamotrigine and carbamazepine monotherapy. Epilepsia 1995;36 (Suppl 3):S186‐7.
- Gillham R, Kane K, Bryant‐Comstock L, Brodie MJ. A double‐blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy with health‐related quality of life as an outcome measure. Seizure 2000;9(6):375‐9. - PubMed
-
- Brodie MJ, Richens A, Yuen AWC, for UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Double‐blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. Lancet 1995;345(8948):476‐9. - PubMed
- Gillham R. Use of SEALS, a quality of life instrument, in evaluating lamotrigine and carbamazepine monotherapy. Epilepsia 1995;36 (Suppl 3):S186‐7.
- Gillham R, Kane K, Bryant‐Comstock L, Brodie MJ. A double‐blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy with health‐related quality of life as an outcome measure. Seizure 2000;9(6):375‐9. - PubMed
-
- Brodie MJ, Giorgi L, and the Lamotrigine Elderly Study Group. A multicenter double‐blind randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. Epilepsia 1998;39 (Suppl 6):72, Abstract no: D.08. - PubMed
- Brodie MJ, Overstall PW, Giorgi L. Multicentre, double‐blind, randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. The UK Lamotrigine Elderly Study Group. Epilepsy Research 1999;37(1):81‐7. - PubMed
- Brodie MJ, Read CL, Gillham RA, Sweet RM, Kane K. Lack of neuropsychological effects of lamotrigine compared to carbamazepine as monotherapy. Epilepsia 1999;40 (Suppl 2):94.
-
- Eun SH, Eun BL, Lee JS, Hwang YS, Kim KJ, Lee YM, et al. Effects of lamotrigine on cognition and behavior compared to carbamazepine as monotherapy for children with partial epilepsy. Brain and Development 2012;34(10):818‐23. - PubMed
-
- Gilad R, Sadeh M, Rapoport A, Dabby R, Boaz M, Lampl Y. Monotherapy of lamotrigine versus carbamazepine in patients with post stroke seizure. Clinical Neuropharmacology 2007;30(4):189‐95. - PubMed
References to studies excluded from this review
-
- Baxter L, Cheesbrough A. An open randomised comparison of Lamictal (Lamotrigine) with physicians preferred choice of either valproate or carbamazepine as monotherapy in patients over 12 years of age with newly diagnosed epilepsy. Clinical Summary Report May 1998.
-
- Carmant L, Curtis P, Moorat AM. Switching paediatric patients to monotherapy: efficacy and tolerability for lamotrigine compared with carbamazepine. Epilepsia 2001;42 (Suppl 7):170.
-
- Czapinski P, Terczynski A, Czapinska E. Open randomised comparative study of vigabatrin (VGB) and lamotrigine (LTG) efficacy in monotherapy of patients with drug‐resistant epilepsy with partial complex seizures resistant to carbamazepine. Epilepsia. 1997; Vol. 38 (Suppl 3):35.
- Czapinski P, Terczynski A, Czapinska E. Open randomized comparative study of vigabatrin (VGB) and lamotrigine (LTG) efficacy in monotherapy of patients with drug‐resistant epilepsy with partial complex seizures resistant to carbamazepine (CBZ). Journal of the Neurological Sciences 1997;150 (Suppl):S96.
- Czapinski P, Terczynski A, Czapiska E. Open randomized comparative study of vigabatrin (VGB) and lamotrigine (LTG) efficacy in monotherapy of patients with drug‐resistant epilepsy with partial complex seizures resistant to carbamazepine (CBZ). Journal of Neurology. 1997; Vol. 244 (Suppl 3):S34.
-
- Eun S, Eun B, Lee J, Lee Y, Hwang Y, Kim K, et al. The effects of cognition and behavior of lamotrigine compared to carbamazepine as monotherapy for children with partial epilepsy. Epilepsia 2008;49(Suppl 7):87, Abstract No: 1.200. - PubMed
-
- Fakhoury T, Gazda S, Nanry KP, Hammer AE, Barrett PS. Comparison of monotherapy with lamotrigine versus carbamazepine in patients with uncontrolled epilepsy with a broad spectrum of seizure types. Epilepsia 2000;41 (Suppl 7):107.
References to studies awaiting assessment
-
- Korean Lamotrigine Study Group. An Open, Randomized, Multicenter Comparative Clinical Trial of Lamotrigine and Carbamazepine as Initial Monotherapy in Previously Untreated Epilepsies. Journal of Korean Epilepsy Society 2008;12(1):27‐34.
Additional references
-
- Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia 1999;40(4):502‐6. - PubMed
-
- Brodie MJ, Richens A, Yuen AWC, for UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Double‐blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. Lancet 1995;345(8948):476‐9. - PubMed
-
- Brodie MJ, Dichter MA. Antiepileptic drugs. New England Journal of Medicine 1996;334(3):168‐75. - PubMed
-
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet 1995;346(8968):140‐4. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

