Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development
- PMID: 27841695
- PMCID: PMC5198941
- DOI: 10.1080/15476278.2016.1252887
Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development
Abstract
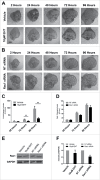
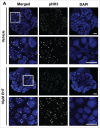
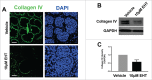
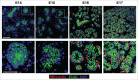
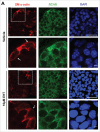
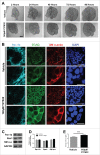
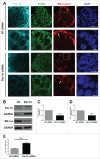
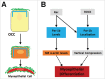
The salivary epithelium initiates as a solid mass of epithelial cells that are organized into a primary bud that undergoes morphogenesis and differentiation to yield bilayered acini consisting of interior secretory acinar cells that are surrounded by contractile myoepithelial cells in mature salivary glands. How the primary bud transitions into acini has not been previously documented. We document here that the outer epithelial cells subsequently undergo a vertical compression as they express smooth muscle α-actin and differentiate into myoepithelial cells. The outermost layer of polarized epithelial cells assemble and organize the basal deposition of basement membrane, which requires basal positioning of the polarity protein, Par-1b. Whether Par-1b is required for the vertical compression and differentiation of the myoepithelial cells is unknown. Following manipulation of Par-1b in salivary gland organ explants, Par-1b-inhibited explants showed both a reduced vertical compression of differentiating myoepithelial cells and reduced levels of smooth muscle α-actin. Rac1 knockdown and inhibition of Rac GTPase function also inhibited branching morphogenesis. Since Rac regulates cellular morphology, we investigated a contribution for Rac in myoepithelial cell differentiation. Inhibition of Rac GTPase activity showed a similar reduction in vertical compression and smooth muscle α-actin levels while decreasing the levels of Par-1b protein and altering its basal localization in the outer cells. Inhibition of ROCK, which is required for basal positioning of Par-1b, resulted in mislocalization of Par-1b and loss of vertical cellular compression, but did not significantly alter levels of smooth muscle α-actin in these cells. Overexpression of Par-1b in the presence of Rac inhibition restored basement membrane protein levels and localization. Our results indicate that the basal localization of Par-1b in the outer epithelial cells is required for myoepithelial cell compression, and Par-1b is required for myoepithelial differentiation, regardless of its localization.
Keywords: Par-1b; ROCK; Rac1; apicobasal polarity; branching morphogenesis; differentiation; myoepithelial cells; submandibular gland.
Figures

Similar articles
-
ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity.Development. 2012 Jan;139(2):411-22. doi: 10.1242/dev.075366. Development. 2012. PMID: 22186730 Free PMC article.
-
TGFβ signaling promotes matrix assembly during mechanosensitive embryonic salivary gland restoration.Matrix Biol. 2015 Apr;43:109-24. doi: 10.1016/j.matbio.2015.01.020. Epub 2015 Jan 31. Matrix Biol. 2015. PMID: 25652203 Free PMC article.
-
Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation.Tissue Eng Part A. 2014 Jun;20(11-12):1632-42. doi: 10.1089/ten.TEA.2013.0515. Epub 2014 Feb 27. Tissue Eng Part A. 2014. PMID: 24410370 Free PMC article.
-
Salivary gland developmental mechanics.Curr Top Dev Biol. 2024;160:1-30. doi: 10.1016/bs.ctdb.2024.05.002. Epub 2024 May 31. Curr Top Dev Biol. 2024. PMID: 38937029 Review.
-
Immunocytochemistry of myoepithelial cells in the salivary glands.Prog Histochem Cytochem. 2003;38(4):343-426. doi: 10.1016/s0079-6336(03)80001-3. Prog Histochem Cytochem. 2003. PMID: 14509196 Review.
Cited by
-
Functional characterization and genomic studies of a novel murine submandibular gland epithelial cell line.PLoS One. 2018 Feb 20;13(2):e0192775. doi: 10.1371/journal.pone.0192775. eCollection 2018. PLoS One. 2018. PMID: 29462154 Free PMC article.
-
Slow hydrogel matrix degradation enhances salivary gland mimetic phenotype.Acta Biomater. 2023 Aug;166:187-200. doi: 10.1016/j.actbio.2023.05.005. Epub 2023 May 5. Acta Biomater. 2023. PMID: 37150277 Free PMC article.
-
Methylmercury-Induced Toxicopathologic Findings in Salivary Glands of Offspring Rats After Gestational and Lactational Exposure.Biol Trace Elem Res. 2021 Aug;199(8):2983-2991. doi: 10.1007/s12011-020-02409-z. Epub 2020 Oct 3. Biol Trace Elem Res. 2021. PMID: 33009984
-
Maternal Fluoride Exposure Exerts Different Toxicity Patterns in Parotid and Submandibular Glands of Offspring Rats.Int J Mol Sci. 2022 Jun 29;23(13):7217. doi: 10.3390/ijms23137217. Int J Mol Sci. 2022. PMID: 35806221 Free PMC article.
-
Bioengineering in salivary gland regeneration.J Biomed Sci. 2022 Jun 6;29(1):35. doi: 10.1186/s12929-022-00819-w. J Biomed Sci. 2022. PMID: 35668440 Free PMC article. Review.
References
-
- Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation 2006; 74:349-64; PMID:16916374; http://dx.doi.org/10.1111/j.1432-0436.2006.00088.x - DOI - PubMed
-
- Tucker AS. Salivary gland development. Semin Cell Dev Biol 2007; 18:237-44; PMID:17336109; http://dx.doi.org/10.1016/j.semcdb.2007.01.006 - DOI - PubMed
-
- Grobstein C. Morphogenetic interaction between embryonic mouse tissues separated by a membrane filter. Nature 1953; 172(4384):869-70; PMID:1311121916613830 - PubMed
-
- Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 2006; 133:1611-24; PMID:16613830 - PubMed
-
- Sakai T. Epithelial branching morphogenesis of salivary gland:exploration of new functional regulators. J Med Investig 2009; 56:234-8; http://dx.doi.org/10.2152/jmi.56.234 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
