Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys
- PMID: 27841870
- PMCID: PMC5145754
- DOI: 10.1038/nature20583
Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys
Abstract
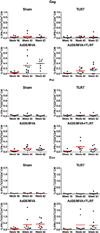
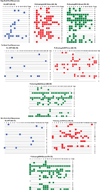
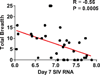
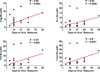
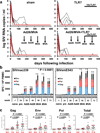
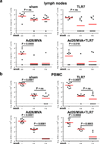
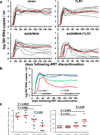
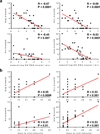
The development of immunologic interventions that can target the viral reservoir in HIV-1-infected individuals is a major goal of HIV-1 research. However, little evidence exists that the viral reservoir can be sufficiently targeted to improve virologic control following discontinuation of antiretroviral therapy. Here we show that therapeutic vaccination with Ad26/MVA (recombinant adenovirus serotype 26 (Ad26) prime, modified vaccinia Ankara (MVA) boost) and stimulation of TLR7 (Toll-like receptor 7) improves virologic control and delays viral rebound following discontinuation of antiretroviral therapy in SIV-infected rhesus monkeys that began antiretroviral therapy during acute infection. Therapeutic vaccination with Ad26/MVA resulted in a marked increase in the magnitude and breadth of SIV-specific cellular immune responses in virologically suppressed, SIV-infected monkeys. TLR7 agonist administration led to innate immune stimulation and cellular immune activation. The combination of Ad26/MVA vaccination and TLR7 stimulation resulted in decreased levels of viral DNA in lymph nodes and peripheral blood, and improved virologic control and delayed viral rebound following discontinuation of antiretroviral therapy. The breadth of cellular immune responses correlated inversely with set point viral loads and correlated directly with time to viral rebound. These data demonstrate the potential of therapeutic vaccination combined with innate immune stimulation as a strategy aimed at a functional cure for HIV-1 infection.
Figures





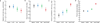
References
-
- Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. - PubMed

