Androgen Receptor in the Ovary Theca Cells Plays a Critical Role in Androgen-Induced Reproductive Dysfunction
- PMID: 27841936
- PMCID: PMC5412974
- DOI: 10.1210/en.2016-1608
Androgen Receptor in the Ovary Theca Cells Plays a Critical Role in Androgen-Induced Reproductive Dysfunction
Abstract
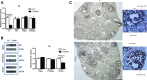
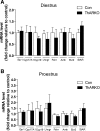
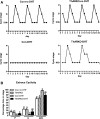
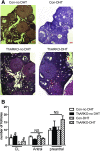
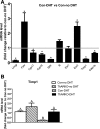
Androgen and its receptor (AR) play a critical role in reproductive function under both physiological and pathophysiological conditions. Female AR global knockout mice are subfertile due to both neuroendocrine and ovarian defects. Female offspring from prenatally androgenized heterozygous AR pregnant mice showed rescued estrous cyclicity and fertility. Ar is expressed in granulosa cells, theca interstitial cells, and oocytes in the ovary. We created mice with theca-specific deletion of Ar (ThARKO) by crossing Cyp17-iCre mice that express Cre recombinase under cytochrome P450 17A1 (Cyp17) promoter with Arfl/fl mice. ThARKO mice exhibited no significant differences in pubertal onset or fertility compared with control littermates, and neither estrogen or testosterone levels were different between these groups. Therefore, Ar expression in theca cells likely does not influence fertility nor androgen levels in female mice. We then tested the role of AR in theca cells under hyperandrogenemic condition. After treatment with a pathophysiological level of dihydrotestosterone (DHT), control mice (control-DHT) showed acyclicity and infertility. However, estrous cycles and fertility were altered to a significantly less degree in ThARKO-DHT mice than in control-DHT mice. Messenger RNA (mRNA) levels of Lhcgr (luteinizing hormone receptor) and Timp1 (tissue inhibitor of metalloproteinase 1, and inhibitor of matrix metalloproteinase) were significantly lower in control-DHT ovary compared with control-no DHT ovaries, whereas mRNA levels of Fshr (follicle-stimulating hormone receptor) were significantly higher. Timp1 gene expression was comparable in the ThARKO-DHT and the control-no DHT ovary. We speculate that the preserved level of Timp1 in ThARKO-DHT mice contributes to retained reproductive function.
Copyright © 2017 by the Endocrine Society.
Figures



References
-
- Walters KA. Role of androgens in normal and pathological ovarian function. Reproduction. 2015;149(4):R193–R218. - PubMed
-
- Walters KA, McTavish KJ, Seneviratne MG, Jimenez M, McMahon AC, Allan CM, Salamonsen LA, Handelsman DJ. Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function. Endocrinology. 2009;150(7):3274–3282. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

