Engulfed cadherin fingers are polarized junctional structures between collectively migrating endothelial cells
- PMID: 27842057
- PMCID: PMC6159904
- DOI: 10.1038/ncb3438
Engulfed cadherin fingers are polarized junctional structures between collectively migrating endothelial cells
Abstract
The development and maintenance of tissues requires collective cell movement, during which neighbouring cells coordinate the polarity of their migration machineries. Here, we ask how polarity signals are transmitted from one cell to another across symmetrical cadherin junctions, during collective migration. We demonstrate that collectively migrating endothelial cells have polarized VE-cadherin-rich membrane protrusions, 'cadherin fingers', which leading cells extend from their rear and follower cells engulf at their front, thereby generating opposite membrane curvatures and asymmetric recruitment of curvature-sensing proteins. In follower cells, engulfment of cadherin fingers occurs along with the formation of a lamellipodia-like zone with low actomyosin contractility, and requires VE-cadherin/catenin complexes and Arp2/3-driven actin polymerization. Lateral accumulation of cadherin fingers in follower cells precedes turning, and increased actomyosin contractility can initiate cadherin finger extension as well as engulfment by a neighbouring cell, to promote follower behaviour. We propose that cadherin fingers serve as guidance cues that direct collective cell migration.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures


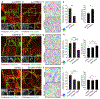

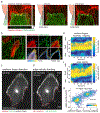
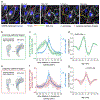
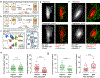
Comment in
-
Cell migration: Let your fingers do the walking.Nat Rev Mol Cell Biol. 2017 Jan;18(1):2-3. doi: 10.1038/nrm.2016.160. Epub 2016 Nov 30. Nat Rev Mol Cell Biol. 2017. PMID: 27899823 No abstract available.
References
-
- Friedl P & Gilmour D Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol 10, 445–57 (2009). - PubMed
-
- Mayor R & Etienne-Manneville S The front and rear of collective cell migration. Nat. Rev. Mol. Cell Biol 17, 97–109 (2016). - PubMed
-
- Reffay M et al. Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat. Cell Biol 16, 217–223 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

