Developmentally programmed germ cell remodelling by endodermal cell cannibalism
- PMID: 27842058
- PMCID: PMC5129868
- DOI: 10.1038/ncb3439
Developmentally programmed germ cell remodelling by endodermal cell cannibalism
Abstract
Primordial germ cells (PGCs) in many species associate intimately with endodermal cells, but the significance of such interactions is largely unexplored. Here, we show that Caenorhabditis elegans PGCs form lobes that are removed and digested by endodermal cells, dramatically altering PGC size and mitochondrial content. We demonstrate that endodermal cells do not scavenge lobes PGCs shed, but rather, actively remove lobes from the cell body. CED-10 (Rac)-induced actin, DYN-1 (dynamin) and LST-4 (SNX9) transiently surround lobe necks and are required within endodermal cells for lobe scission, suggesting that scission occurs through a mechanism resembling vesicle endocytosis. These findings reveal an unexpected role for endoderm in altering the contents of embryonic PGCs, and define a form of developmentally programmed cell remodelling involving intercellular cannibalism. Active roles for engulfing cells have been proposed in several neuronal remodelling events, suggesting that intercellular cannibalism may be a more widespread method used to shape cells than previously thought.
Figures
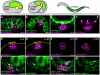
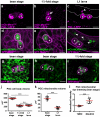


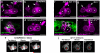

Comment in
-
Germ Cells Get by with a Little Cannibalistic Help from Their Friends.Dev Cell. 2016 Dec 19;39(6):631-633. doi: 10.1016/j.devcel.2016.12.003. Dev Cell. 2016. PMID: 27997820
-
Embryonic Trogocytosis: Neighborly Nibbling during Development.Curr Biol. 2017 Jan 23;27(2):R68-R70. doi: 10.1016/j.cub.2016.11.043. Curr Biol. 2017. PMID: 28118592
References
-
- Cinalli RM, Rangan P, Lehmann R. Germ cells are forever. Cell. 2008;132:559–562. - PubMed
-
- Seydoux G, Braun RE. Pathway to totipotency: lessons from germ cells. Cell. 2006;127:891–904. - PubMed
-
- Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240:488–498. - PubMed
-
- Takamura K, Fujimura M, Yamaguchi Y. Primordial germ cells originate from the endodermal strand cells in the ascidian Ciona intestinalis. Dev Genes Evol. 2002;212:11–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

