Association of Amyloid Pathology With Myelin Alteration in Preclinical Alzheimer Disease
- PMID: 27842175
- PMCID: PMC5195903
- DOI: 10.1001/jamaneurol.2016.3232
Association of Amyloid Pathology With Myelin Alteration in Preclinical Alzheimer Disease
Abstract
Importance: The accumulation of aggregated β-amyloid and tau proteins into plaques and tangles is a central feature of Alzheimer disease (AD). While plaque and tangle accumulation likely contributes to neuron and synapse loss, disease-related changes to oligodendrocytes and myelin are also suspected of playing a role in development of AD dementia. Still, to our knowledge, little is known about AD-related myelin changes, and even when present, they are often regarded as secondary to concomitant arteriosclerosis or related to aging.
Objective: To assess associations between hallmark AD pathology and novel quantitative neuroimaging markers while being sensitive to white matter myelin content.
Design, setting, and participants: Magnetic resonance imaging was performed at an academic research neuroimaging center on a cohort of 71 cognitively asymptomatic adults enriched for AD risk. Lumbar punctures were performed and assayed for cerebrospinal fluid (CSF) biomarkers of AD pathology, including β-amyloid 42, total tau protein, phosphorylated tau 181, and soluble amyloid precursor protein. We measured whole-brain longitudinal and transverse relaxation rates as well as the myelin water fraction from each of these individuals.
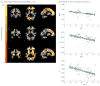
Main outcomes and measures: Automated brain mapping algorithms and statistical models were used to evaluate the relationships between age, CSF biomarkers of AD pathology, and quantitative magnetic resonance imaging relaxometry measures, including the longitudinal and transverse relaxation rates and the myelin water fraction.
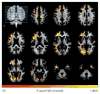
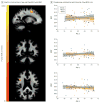
Results: The mean (SD) age for the 19 male participants and 52 female participants in the study was 61.6 (6.4) years. Widespread age-related changes to myelin were observed across the brain, particularly in late myelinating brain regions such as frontal white matter and the genu of the corpus callosum. Quantitative relaxometry measures were negatively associated with levels of CSF biomarkers across brain white matter and in areas preferentially affected in AD. Furthermore, significant age-by-biomarker interactions were observed between myelin water fraction and phosphorylated tau 181/β-amyloid 42, suggesting that phosphorylated tau 181/β-amyloid 42 levels modulate age-related changes in myelin water fraction.
Conclusions and relevance: These findings suggest amyloid pathologies significantly influence white matter and that these abnormalities may signify an early feature of the disease process. We expect that clarifying the nature of myelin damage in preclinical AD may be informative on the disease's course and lead to new markers of efficacy for prevention and treatment trials.
Conflict of interest statement
Disclosures: Dr Carlsson serves as a site principal investigator for a study that is jointly funded by the National Institutes of Health and Eli Lilly and Company and receives funding from the US Department of Veterans Affairs through the Veterans Affairs Merit grant program. Dr Blennow has served as a consultant at and on advisory boards for IBL International, Fujirebio Europe, Eli Lilly, and Novartis. No other disclosures were reported.
Figures

Comment in
-
An Emerging Role for Imaging White Matter in the Preclinical Risk for Alzheimer Disease: Linking β-Amyloid to Myelin.JAMA Neurol. 2017 Jan 1;74(1):17-19. doi: 10.1001/jamaneurol.2016.4123. JAMA Neurol. 2017. PMID: 27842149 No abstract available.
References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

