EpCAM as multi-tumour target for near-infrared fluorescence guided surgery
- PMID: 27842504
- PMCID: PMC5109830
- DOI: 10.1186/s12885-016-2932-7
EpCAM as multi-tumour target for near-infrared fluorescence guided surgery
Abstract
Background: Evaluation of resection margins during cancer surgery can be challenging, often resulting in incomplete tumour removal. Fluorescence-guided surgery (FGS) aims to aid the surgeon to visualize tumours and resection margins during surgery. FGS relies on a clinically applicable imaging system in combination with a specific tumour-targeting contrast agent. In this study EpCAM (epithelial cell adhesion molecule) is evaluated as target for FGS in combination with the novel Artemis imaging system.
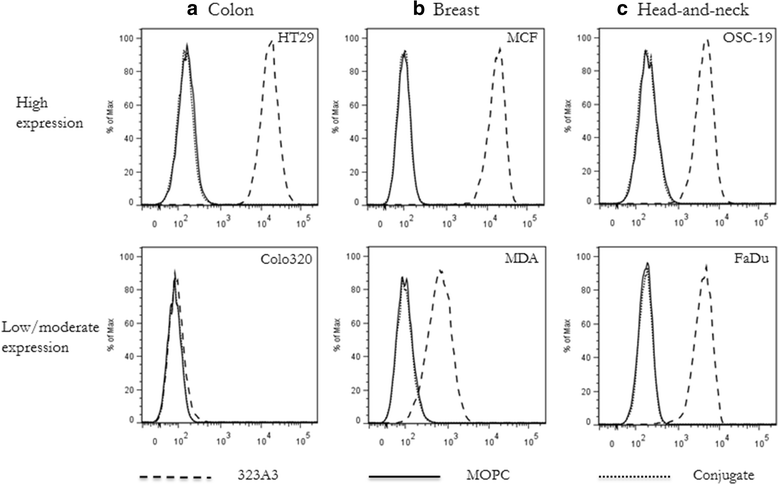
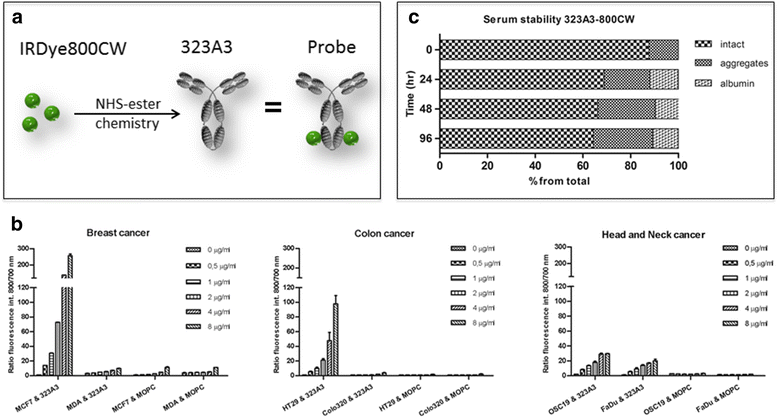
Methods: The NIR fluorophore IRDye800CW was conjugated to the well-established EpCAM specific monoclonal antibody 323/A3 and an isotype IgG1 as control. The anti-EpCAM/800CW conjugate was stable in serum and showed preserved binding capacity as evaluated on EpCAM positive and negative cell lines, using flow cytometry and cell-based plate assays. Four clinically relevant orthotopic tumour models, i.e. colorectal cancer, breast cancer, head and neck cancer, and peritonitis carcinomatosa, were used to evaluate the performance of the anti-EpCAM agent with the clinically validated Artemis imaging system. The Pearl Impulse small animal imaging system was used as reference. The specificity of the NIRF signal was confirmed using bioluminescence imaging and green-fluorescent protein.
Results: All tumour types could clearly be delineated and resected 72 h after injection of the imaging agent. Using NIRF imaging millimetre sized tumour nodules were detected that were invisible for the naked eye. Fluorescence microscopy demonstrated the distribution and tumour specificity of the anti-EpCAM agent.
Conclusions: This study shows the potential of an EpCAM specific NIR-fluorescent agent in combination with a clinically validated intraoperative imaging system to visualize various tumours during surgery.
Keywords: Epithelial cell adhesion molecule; Image-guided surgery; Imaging agent; Near-infrared fluorescence; Optical imaging.
Figures




Similar articles
-
Preclinical evaluation of EpCAM-binding designed ankyrin repeat proteins (DARPins) as targeting moieties for bimodal near-infrared fluorescence and photoacoustic imaging of cancer.Eur J Nucl Med Mol Imaging. 2024 Jul;51(8):2179-2192. doi: 10.1007/s00259-023-06407-w. Epub 2023 Aug 29. Eur J Nucl Med Mol Imaging. 2024. PMID: 37642704 Free PMC article.
-
Fluorescence-guided tumor detection with a novel anti-EpCAM targeted antibody fragment: Preclinical validation.Surg Oncol. 2019 Mar;28:1-8. doi: 10.1016/j.suronc.2018.10.004. Epub 2018 Oct 23. Surg Oncol. 2019. PMID: 30851880
-
Comparison of mAbs targeting epithelial cell adhesion molecule for the detection of prostate cancer lymph node metastases with multimodal contrast agents: quantitative small-animal PET/CT and NIRF.J Nucl Med. 2012 Sep;53(9):1427-37. doi: 10.2967/jnumed.112.106302. Epub 2012 Aug 7. J Nucl Med. 2012. PMID: 22872743
-
Revisiting the Roles of Pro-Metastatic EpCAM in Cancer.Biomolecules. 2020 Feb 7;10(2):255. doi: 10.3390/biom10020255. Biomolecules. 2020. PMID: 32046162 Free PMC article. Review.
-
Quicker, deeper and stronger imaging: A review of tumor-targeted, near-infrared fluorescent dyes for fluorescence guided surgery in the preclinical and clinical stages.Eur J Pharm Biopharm. 2020 Jul;152:123-143. doi: 10.1016/j.ejpb.2020.05.002. Epub 2020 May 8. Eur J Pharm Biopharm. 2020. PMID: 32437752 Review.
Cited by
-
Implication of Different Tumor Biomarkers in Drug Resistance and Invasiveness in Primary and Metastatic Colorectal Cancer Cell Lines.Biomedicines. 2022 May 6;10(5):1083. doi: 10.3390/biomedicines10051083. Biomedicines. 2022. PMID: 35625820 Free PMC article.
-
Fluorescence Molecular Targeting of Colon Cancer to Visualize the Invisible.Cells. 2022 Jan 12;11(2):249. doi: 10.3390/cells11020249. Cells. 2022. PMID: 35053365 Free PMC article. Review.
-
The Application of Heptamethine Cyanine Dye DZ-1 and Indocyanine Green for Imaging and Targeting in Xenograft Models of Hepatocellular Carcinoma.Int J Mol Sci. 2017 Jun 21;18(6):1332. doi: 10.3390/ijms18061332. Int J Mol Sci. 2017. PMID: 28635650 Free PMC article.
-
Fluorescence-guided tumor visualization of colorectal cancer using tumor-initiating probe yellow in preclinical models.Sci Rep. 2024 Nov 6;14(1):26946. doi: 10.1038/s41598-024-76312-1. Sci Rep. 2024. PMID: 39505985 Free PMC article.
-
Potential Probes for Targeted Intraoperative Fluorescence Imaging in Gastric Cancer.Cancers (Basel). 2024 Dec 12;16(24):4141. doi: 10.3390/cancers16244141. Cancers (Basel). 2024. PMID: 39766041 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

