Rubidium and potassium levels are altered in Alzheimer's disease brain and blood but not in cerebrospinal fluid
- PMID: 27842602
- PMCID: PMC5109650
- DOI: 10.1186/s40478-016-0390-8
Rubidium and potassium levels are altered in Alzheimer's disease brain and blood but not in cerebrospinal fluid
Abstract
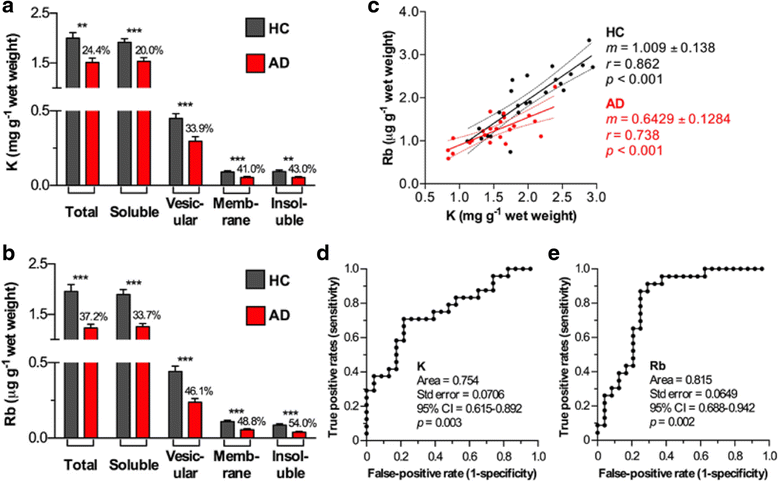
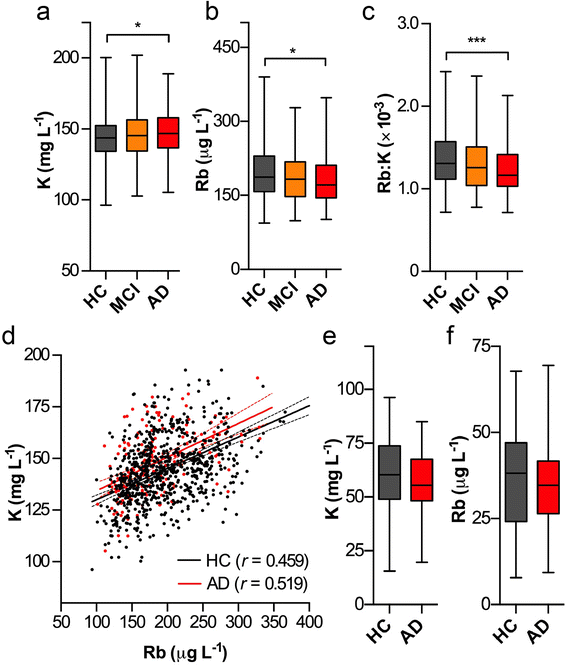
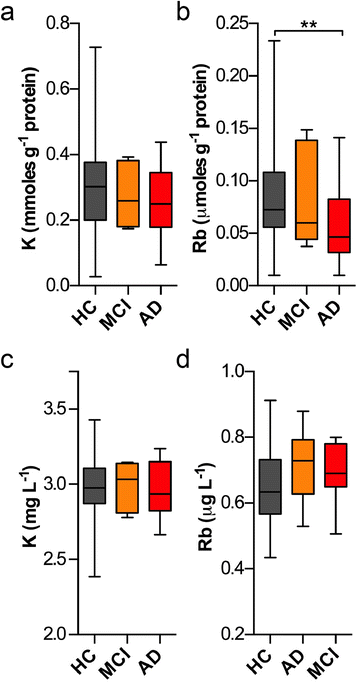
Loss of intracellular compartmentalization of potassium is a biochemical feature of Alzheimer's disease indicating a loss of membrane integrity and mitochondrial dysfunction. We examined potassium and rubidium (a biological proxy for potassium) in brain tissue, blood fractions and cerebrospinal fluid from Alzheimer's disease and healthy control subjects to investigate the diagnostic potential of these two metal ions. We found that both potassium and rubidium levels were significantly decreased across all intracellular compartments in the Alzheimer's disease brain. Serum from over 1000 participants in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL), showed minor changes according to disease state. Potassium and rubidium levels in erythrocytes and cerebrospinal fluid were not significantly different according to disease state, and rubidium was slightly decreased in Alzheimer's disease patients compared to healthy controls. Our data provides evidence that contrasts the hypothesized disruption of the blood-brain barrier in Alzheimer's disease, with the systemic decrease in cortical potassium and rubidium levels suggesting influx of ions from the blood is minimal and that the observed changes are more likely indicative of an internal energy crisis within the brain. These findings may be the basis for potential diagnostic imaging studies using radioactive potassium and rubidium tracers.
Figures
References
-
- Angelova PR, Abramov AY. Alpha-synuclein and beta-amyloid – different targets, same players: calcium, free radicals and mitochondria in the mechanism of neurodegeneration. Biochem Biophys Res Commun. 2016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

